Green Aluminium: The Scientific and Engineering Foundations of Sustainable Light Metals
The Aluminium Paradox when it comes to Sustainability
Aluminium is both a sustainability enabler and a sustainability problem.
Its low density (2.70 g cm⁻³), high specific strength, and excellent corrosion resistance make it indispensable for lightweight transport,
electrification, and energy-efficient construction. Every tonne of aluminium used in vehicles typically saves 20–25 t CO₂ over the vehicle’s lifetime through reduced fuel consumption. Yet, the
production of primary aluminium from bauxite remains one of the most energy- and emission-intensive processes in all of metallurgy.
Globally, aluminium accounts for ~3 % of total anthropogenic greenhouse gas emissions and ~1 % of total global energy use.
The Hall–Héroult electrolysis process requires roughly 13–15 MWh t⁻¹ Al, generating
12–16.5 t CO₂-eq t⁻¹ Al when powered by fossil electricity. Roughly two-thirds of these
emissions stem from carbon-based electricity and carbon anode oxidation. The global aluminium industry thus emits around 1.1 Gt CO₂-eq yr⁻¹, comparable to the entire aviation sector.
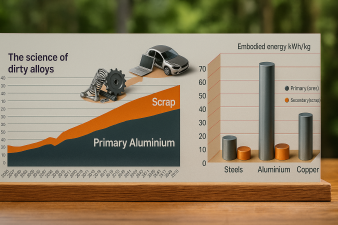
By contrast, re-melting aluminium scrap requires only 2.8 kWh kg⁻¹, i.e. ≈ 5 % of the primary energy input, and emits merely 0.6 kg CO₂ kg⁻¹ Al. These metrics make aluminium uniquely suited for circularity — if the metallurgical challenges of impurity management and alloy compatibility can be solved.
1-s2.0-S1359645425002241-main.pdf
PDF-Dokument [4.6 MB]
The Roles of Manufacturing Efficiency and Near‑Net Processes in Green Aluminiun Making
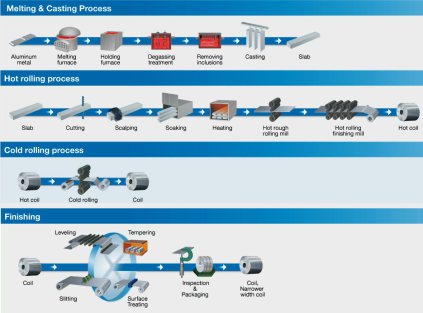
Approximately 40 % of aluminium mass input is lost along conventional casting, forming, and fabrication chains. The combined energy penalty of these yield losses approaches 5 % of sectoral emissions. Thin‑strip and twin‑roll casting reduce the as‑cast thickness from > 150 mm to 1–10 mm, eliminating multiple remelt‑ and reheat‑intensive steps. The solidification velocity suppresses segregation and tolerates higher impurity levels, easing integration of recycled feedstock. Further downstream, additive and near‑net‑shape manufacturing may localize production but remain sustainability‑neutral unless powder recovery and oxidation losses are minimized. Overall gains require coupling near‑net processes with renewable‑electric heat sources to decarbonize both electricity and fossil‑fired re‑melts.
Sustainable Aluminium from Ore to Metal: Limits of Primary Synthesis and green alternatives
Primary aluminium is derived from bauxite (gibbsite, boehmite, diaspore), refined via the Bayer process into alumina and reduced electrolytically in molten cryolite (Na₃AlF₆) at ≈ 950 °C.
Key environmental burdens arise from:
- Electricity demand: 13–15 MWh t⁻¹ metal; CO₂ intensity depends on grid mix.
- Carbon anode oxidation: ~1.5 t C t⁻¹ Al consumed, directly producing CO₂.
- PFC (CF₄, C₂F₆) emissions: 1–3 kg CO₂-eq kg⁻¹ Al during anode effects.
- Red-mud generation: ≈ 1.5 t waste t⁻¹ Al₂O₃, containing caustic oxides and residual metals.
The Nature analysis by Raabe et al. quantified aluminium’s global burden at 94 Mt
yr⁻¹ production, 13 EJ yr⁻¹ energy use, and 0.7 Gt CO₂ yr⁻¹. Despite hydropower decarbonizing parts of the industry (notably in Canada, Norway, Iceland, China’s Yunnan), over 65 % of
smelting electricity remains fossil-derived.
Inert-anode and non-cryolite electrolytes (e.g. NiFe₂O₄–CuNiO ceramics, AlCl₃-based ionic liquids) are being developed but face issues of corrosion, wetting, and stability at scale. Consequently, the
near-term decarbonization path relies on renewable electricity integration and maximized scrap utilization rather than wholesale process substitution.
Raabe et al Nature 2019 sustainability o[...]
PDF-Dokument [2.2 MB]
Decarbonized electrolysis and process redesign
The transition to green aluminium is governed by two complementary axes: substitution of the carbon energy input with renewable electricity, and substitution of the carbon anode with inert or oxygen‑evolving materials. The first is already underway in hydropower‑based smelters operating at grid factors < 2 kg CO₂ kg⁻¹ Al compared with > 10 kg CO₂ kg⁻¹ Al for coal‑sourced grids. The second axis involves design of stable oxide or cermet anodes—NiFe₂O₄, CuNiO, or doped spinel systems—that sustain oxygen evolution in cryolite melts without consumable carbon. Laboratory lifetimes now exceed 1000 h at > 0.8 A cm⁻², bringing technical readiness to pilot demonstration. Combined, these measures can cut direct process emissions by > 95 %, limiting residual footprint to electricity sourcing and alumina calcination.
A sustainable Pathway towards Green Aluminium: Secondary Synthesis and the Scrap Economy
About 35 % of global aluminium currently originates from scrap; this share is projected to exceed 50 % by 2050.
Scrap streams are classified as:
- Pre-consumer scrap: clean, single-alloy, production residues — readily recyclable.
- Post-consumer scrap: mixed, contaminated alloys from end-of-life vehicles, buildings, and packaging — compositionally “dirty.”
While aluminium is thermodynamically recyclable without degradation, chemical complexity is the main barrier. Post-consumer scrap contains variable concentrations of Fe, Cu, Zn, Ni, Cr, Mn, and Si. Most of these have solubilities in aluminium below 0.05 wt % at 660 °C. Excess solute forms brittle, acicular intermetallic compounds such as β-Al₅FeSi, which severely reduce ductility, corrosion resistance, and surface finish.
Traditional practice mitigates this by downcycling — routing contaminated scrap to cast alloys that tolerate higher impurity loads (~2–3 wt % Fe). However, the demand for wrought
alloys (sheets, extrusions) in automotive and battery applications is rising, while casting markets stagnate.
Thus, the future of green aluminium depends on developing scrap-tolerant, compositionally robust alloys that can re-absorb post-consumer scrap without dilution by virgin metal.
What is meant by the Science of “Dirty Alloys” (SoDA)?
due to its low density, producing it from ores is very energy-intensive. Recycling it shifts the balance towards higher sustainability, because the energy needed to melt aluminum from scrap is only about 5% of that consumed in ore reduction. The amount of aluminum available for recycling is estimated to double by 2050. This offers an opportunity to bring the metallurgical sector closer to a circular economy. A challenge is that large amounts of scrap are post-consumer scrap, containing high levels of elemental contamination. This has to be taken into account in more sustainable alloy design strategies. A “green aluminum” trend has already triggered a new trading platform for low-carbon aluminum at the London Metal Exchange (2020). The trend may lead to limits on the use of less-sustain
OPEN ACCESS - Making sustainable aluminu[...]
PDF-Dokument [4.8 MB]
We introduced the term Science of Dirty Alloys (SoDA) to describe this emerging field of
scrap-compatible alloy design.
Its goal: transform contamination from a liability into a controllable design parameter.
SoDA research spans three main scientific frontiers:
(i) Thermodynamic Multicomponent Complexity
Designing alloys from heterogeneous scrap requires navigating compositional spaces of up to 10–15 elements.
CALPHAD databases, traditionally parameterized for binary or ternary equilibria, must be extended to include metastable and intermetallic phases rich in Fe, Ni, Cu, and Zn. Modified phase diagrams
and computed solidification paths help predict the precipitation of β-Al₅FeSi, α-Al(FeMn)Si, or Al₇Cu₂Fe phases.
For instance, Mn additions (~0.4–0.8 wt %) can transform needle-like β-Al₅FeSi into more benign, globular α-Al(FeMn)Si morphologies, improving formability and toughness.
(ii) Microstructure-Scale Mechanisms
Tramp elements perturb every stage of microstructural evolution:
- Vacancy interactions: Fe or Cu act as vacancy traps, slowing diffusion and altering precipitation kinetics.
- Precipitate poisoning: Cu or Zn sequester Mg or Si, suppressing strengthening phases (β″, Q′).
- Grain-boundary segregation: promotes intergranular corrosion and hot-shortness.
- Intermetallic morphology: affects fatigue initiation and surface finish.
Advanced 4D-STEM, atom-probe tomography, and in-situ synchrotron diffraction reveal these nanoscale effects and guide countermeasures such as controlled solidification rates (10²–10⁴ K s⁻¹ in twin-roll casting) or optimized homogenization profiles.
(iii) Process and Alloy Engineering
Scientific levers for impurity tolerance include:
- Rapid solidification and strip casting to refine intermetallics.
- Thermomechanical routes that dissolve or redistribute deleterious phases.
- Micro-alloying with elements such as Cr, Zr, Ti, or Sc to stabilize dispersoids.
- Machine-learning-assisted process control, linking sensor data to CALPHAD and ICME models for real-time quality assurance.
Together these approaches constitute a metallurgical toolbox for upgrading post-consumer scrap into high-performance alloys — effectively upcycling rather than downcycling.
Sustainable Alloy Design for Recycled Aluminium: Quantitative Criteria
Sustainability in aluminium cannot be defined by recycled content alone; it requires full life-cycle assessment (LCA) covering energy, emissions, material efficiency, and ethical
sourcing.
Quantitatively, major leverage points include:
|
Measure |
Typical Reduction |
Technology Readiness |
|---|---|---|
|
Renewable electricity for electrolysis |
–60 % CO₂ intensity |
High (TRL 9) |
|
High-scrap secondary synthesis |
–90 % energy use |
High |
|
Thin-strip / near-net-shape casting |
–20–30 % energy |
Medium |
|
Compositionally lean alloys |
–5–15 % embodied energy |
Medium |
|
Hydrogen-based heating & remelting |
–10–20 % CO₂ |
Emerging |
In practice, green aluminium combines several of these measures: hydro-powered smelting (as in Alcoa’s “Elysis” inert-anode project), integration of 70–90 % scrap, and digital LCA verification
(“material passports”).
The London Metal Exchange has since 2020 established a dedicated low-carbon aluminium trading platform, defining “green aluminium” as < 4 t CO₂-eq t⁻¹ metal at the smelter gate.
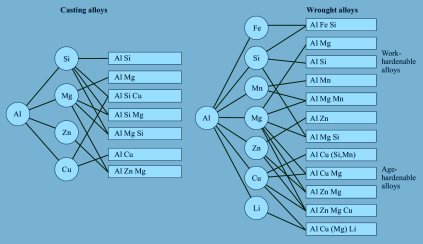
Green Aluminium Alloy Systematics and Scrap Tolerance across the Different Aluminium Classes
Commercial aluminium alloys differ widely in their impurity sensitivity:
- 1xxx series (pure Al): high conductivity, extremely sensitive to Fe/Cu; limited scrap tolerance.
- 3xxx series (Al–Mn): moderately tolerant; Mn mitigates Fe effects.
- 5xxx series (Al–Mg): good tolerance; used for automotive sheets.
- 6xxx series (Al–Mg–Si): age-hardenable, sensitive to Cu/Zn contamination.
- 7xxx series (Al–Zn–Mg–Cu): high strength but narrow composition window.
Designing “unialloys” or “crossover alloys” merges these families, broadening composition windows while maintaining acceptable property envelopes. Such alloys may exhibit tensile strengths from 300 to 550 MPa purely through microstructural tuning, without tight chemical control.
The guiding principle is achieve performance by microstructure rather than composition, since microstructure can be re-engineered during recycling, whereas composition is conserved.
Metallurgical Measures and Metrics Towards a Circular Aluminium Economy
A sustainable aluminium cycle requires coupling metallurgical innovation with digital and logistical infrastructures:
- Advanced scrap sorting: laser-induced breakdown spectroscopy (LIBS) and AI-based classification of mixed scrap.
- Digital material passports: trace chemical composition and processing history across product life cycles.
- Closed-loop supply chains: automotive and packaging sectors reintegrating their own scrap.
- Hydrogen-based re-melting and pre-heating: replacing natural gas in recycling furnaces.
- LCA-verified certification: quantifying embodied energy (MJ kg⁻¹) and CO₂ footprint (kg CO₂ kg⁻¹ Al) for each batch.
Quantitatively, a fully circular aluminium system using 80 % scrap and renewable electricity could reduce the global sector’s emissions from ~1.1 Gt yr⁻¹ to < 0.2 Gt yr⁻¹, corresponding to a > 80 % decarbonization potential.
The transition to green aluminium will not be achieved by a single technology, but by the integration of four pillars:
- Low-carbon primary synthesis – inert anodes, hydropower, hydrogen-heated refining.
- High-scrap secondary synthesis – impurity-tolerant alloys, AI-guided remelting.
- Circular alloy design – compositionally lean, microstructure-driven “dirty alloys.”
- Digital traceability and certification – data-driven LCAs and transparent supply chains.
In the terminology of Raabe et al., the goal is to replace composition control by microstructure control, and energy intensity by information intensity.
By merging thermodynamics, kinetics, and data science, green aluminium metallurgy can transform one of humanity’s largest industrial emitters into a cornerstone of the circular, fossil-free
economy.