Elucidating the microstructure evolution during hydrogen-based direct reduction via a case study of single crystal hematite
This study examines the fundamental microstructural evolution during hydrogen-based direct reduction (HyDR) of dense single-crystal hematite (Fe₂O₃) at 700 °C,
with the aim of disentangling the intrinsic reduction mechanisms from the complexities of industrial pellets. Unlike sintered polycrystalline pellets—which contain inherited porosity, grain
boundaries, and gangue phases—single crystals (SCs) provide a clean model to probe reduction front morphology, phase transformations, and crystallographic effects.
Kinetics and macro-scale behaviour.
Thermogravimetric analysis hlped us to compare dense SCs with porous industrial pellets (~30 % porosity). Pellets reduced faster in early stages—reaching 55 % metallisation in 6 min versus 35 % for
SCs—due to pre-existing pore networks enabling rapid gas transport. However, SCs approached similar final conversions (96 % vs. 91 %), with the last few percent hindered by oxide entrapment in dense
Fe. For SCs, the reduction rate plateaued at ~0.1 s⁻¹ up to ~30 % metallisation, indicating overlapping phase transformations (Fe₂O₃→Fe₃O₄→Fe₁₋ₓO→α-Fe), contrasting with the sequential behaviour in
pellets.
Crystallographic facet dependence
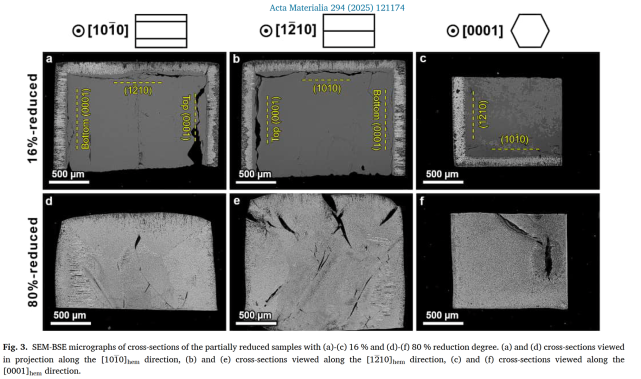
Partial reduction experiments (16 % and 80 %) on SCs with surfaces parallel to (0001)ₕₑₘ, (10-10)ₕₑₘ, and (12-10)ₕₑₘ planes revealed initial shrinking-core behaviour, with reduction fronts advancing
~10 % faster perpendicular to (0001) facets. At 16 % reduction, a core-shell structure was present; by 80 %, reduction was spatially uniform, with converging fronts and diagonal cracking.
Microstructural analysis showed facet-dependent iron/oxide ratios and porosity: top basal planes had ~64 % Fe and 3 % porosity in reduced layers, whereas (10-10) planes had ~61 % Fe and 11 %
porosity.
Hierarchical porosity and “cell” morphology
Near the hematite/magnetite interface, reduction produced a “cell-like” magnetite structure: finely nanoporous, strongly textured interiors (~1–2 µm across) enclosed by coarser, weakly textured “cell
walls” with larger pores. These walls served as preferential nucleation sites for wüstite and α-Fe, which grew inward into the textured interiors. Nanopore channels (~28–35 nm diameter, ~70–80 nm
spacing) extended 2–4 µm from the interface before coarsening; the bottom basal plane showed ~15–20 % larger pores, likely from altered gas flow.
Phase transformation sequence and retention
Despite thermodynamic expectations, Fe often formed adjacent to magnetite without an intervening wüstite layer, suggesting localised bypassing of the grain-model sequence. Magnetite and wüstite
exhibited cube-on-cube orientation relationships, with no grain refinement during Fe₃O₄→Fe₁₋ₓO transformation. After 80 % overall reduction, ~30 % of oxide phases remained as elongated clusters
trapped within Fe, impeding oxygen diffusion and slowing final reduction.
Porosity–phase correlations
Image analysis across reduced layers showed porosity peaking shortly after Fe nucleation, driven by ~41 % volume contraction in the wüstite→Fe step. Iron fraction maxima and distances from the
hematite/magnetite interface varied: (10-10)/(12-10) facets initiated Fe formation closer to the interface (~10 µm) than basal facets (~20–30 µm).
Crystallographic orientation relationships
Electron backscatter diffraction revealed a dominant (112)ₘₐg//(0001)ₕₑₘ, [110]ₘₐg//[10-10]ₕₑₘ orientation (OR2), typical of porous cellular magnetite at intermediate temperatures, rather than the
Shoji–Nishiyama OR common in lamellar morphologies. OR2 prevalence was consistent across facets, indicating a magnetite growth mode influenced by temperature and interface conditions.
Conclusions
HyDR of dense hematite single crystals proceeds via an initial shrinking-core regime followed by homogeneous reduction facilitated by newly formed pore networks. Reduction rates and microstructures
are facet-dependent, with basal planes reducing faster and producing higher Fe fractions. The cell-like magnetite morphology, hierarchical porosity, and preferential Fe nucleation at coarse-pore
walls are key microstructural motifs. Persistent oxide clusters, facet-specific pore metrics, and crystallographic OR2 dominance provide mechanistic insights relevant to dense regions in industrial
pellets and natural ores, informing optimisation of pellet design and reactor conditions for efficient hydrogen-based steelmaking.
Sustainable hydrogen-based direct reduction (HyDR) of iron oxide is an effective approach to reduce carbon emissions in steel production. As the reduction behaviour is closely related to the microstructure evolution, it is important to understand the microscopic reduction mechanisms. Industrial hematite pellets are microstructurally intricate systems with inherent porosity, defects, and impurities. Therefore, in the present study we investigated the HyDR of single crystal hematite (at 700 ◦ C) to elucidate the reduction behaviour and microstructure evolution in a model system. The reduction kinetics of the single crystal (SC) were compared to those of industrial polycrystalline porous pellets using thermogravimetric analysis. Additional SC samples were prepared such that their faces are
Elucidating microstructure hydrogen-base[...]
PDF-Dokument [2.7 MB]
Details:
Methods
Rectangular hematite SC samples oriented along the (0001), (10–10), and (12–10) planes (3 × 3 × 2 mm³, purity > 99.99%) were reduced in a custom thermogravimetric setup with 10 L h⁻¹ of 99.999 % H₂. Temperature was raised to 700 °C (5 °C s⁻¹) and held isothermally for 3 h. Intermediate reductions (2.5, 4, 15 min → 16 %, 27 %, 80 % reduction) captured transient microstructures. Microstructural and crystallographic analyses used SEM-BSE, electron channeling contrast imaging (ECCI), and EBSD.
Kinetic Behaviour
The SC hematite reduced markedly slower than the porous pellet: after 6 min the pellet achieved 55 % reduction versus 35 % for the SC, due to its inherited pore network facilitating gas transport. Nevertheless, the dense crystal reached 96 % reduction after 3 h. Both systems showed three kinetic regimes corresponding to Fe₂O₃→Fe₃O₄ (fast), Fe₃O₄→Fe₁₋ₓO (intermediate), and Fe₁₋ₓO→Fe (slow, <10⁻³ s⁻¹). The dense SC formed directional cracks enabling localized gas ingress; the final iron product had grains ~1.5 µm compared with 0.8 µm in pellets and an acquired porosity of only ~3 %.
Microstructure Evolution
At 16 % reduction, a clear shrinking-core morphology appeared: a magnetite shell (~130–150 µm thick) surrounding an unreacted hematite core. Reduction proceeded faster (~10 %) normal to (0001) basal planes than to prismatic facets, reflecting anisotropic diffusion. As conversion advanced to 80 %, the shell–core distinction vanished and the microstructure homogenized with converging reduction fronts and residual oxides dispersed in metallic Fe.
Microscopically, hierarchical porosity developed:
-
At the hematite/magnetite interface, nanoscale pore channels (~30 ± 10 nm diameter, ~70 nm spacing, 2–4 µm length) nucleated by vacancy accumulation.
-
These organized into “cell-like” magnetite morphology: fine porous “cell interiors” enclosed by coarse-pored “cell walls”.
-
The cell size depended on facet orientation—1–2 µm for prismatic, 2–5 µm for basal facets—and wall thickness scaled proportionally.
ECCI showed high dislocation density in hematite adjacent to the interface, caused by ~18 % thermal expansion mismatch and transformation strain. Dislocation-assisted vacancy diffusion contributed to pore channel alignment normal to the interface.
Phase Transformations and Orientation Relationships
EBSD revealed a cube-on-cube relationship between magnetite and wüstite and absence of grain refinement during Fe₃O₄→Fe₁₋ₓO. Metallic Fe nucleated predominantly along magnetite cell walls and grew inward into cell interiors, generating 3-D “pipe-like” Fe morphologies enclosing elongated oxide residues. Thus, the reduction path deviated from the ideal grain model—iron often appeared in direct contact with magnetite without an intervening continuous wüstite layer.
The predominant orientation relationship (OR) at the hematite/magnetite interface was
(112)ₘₐg // (0001)ₕₑₘ; [110]ₘₐg // [10–10]ₕₑₘ (so-called OR2), typical for cellular morphologies with incoherent interfaces. The classical Shoji–Nishiyama
OR ((111)ₘₐg // (0001)ₕₑₘ) occurred in < 10 % of interfaces, indicating that at 700 °C the system lies within the transition regime between lamellar (≥ 800 °C) and cellular (≤ 650 °C)
magnetite formation.
Quantitative Porosity and Phase Profiles
Across all facets, porosity rose from ~10 % near the interface to a maximum of ~25 % in regions where iron first appeared, then stabilized around 15–25 %. The highest iron fraction (~45 %) occurred at basal planes, consistent with accelerated reduction there. The onset of metallic iron was located ~10 µm from the interface for prismatic facets and ~20–30 µm for basal ones. Gas-flow asymmetry in the vertical furnace caused the bottom (0001) facet to develop larger pores and oxide/iron streaks.
Mechanistic Interpretation
Reduction proceeds in two stages:
-
Topochemical Stage: a moving reaction front produces magnetite with cellular morphology; pore nucleation and crystallographic anisotropy dominate.
-
Homogeneous Stage: once hematite is consumed, reduction follows pore/grain kinetics, limited by solid-state O-diffusion through Fe and gas-phase H₂/H₂O transport in percolating pores.
Residual oxides (Fe₁₋ₓO : Fe₃O₄ ≈ 2:1) persisted even after 80 % reduction due to encapsulation by iron and diffusion-limited O removal (D_O ≈ 10⁻¹⁴–10⁻¹¹ m² s⁻¹ in Fe vs 10⁻¹⁸ m² s⁻¹ in oxides). Trapped H₂O in closed pores likely caused local re-oxidation and explains the sluggish final Fe₁₋ₓO→Fe step.
Key Conclusions
-
Single-crystal hematite enables isolation of intrinsic reduction mechanisms free from sinter porosity.
-
Despite initial density, a percolating pore network self-organizes, allowing > 90 % reduction after 1 h at 700 °C.
-
Reduction follows a shrinking-core → homogeneous transition, with anisotropic kinetics fastest perpendicular to (0001) planes.
-
Magnetite forms hierarchical cellular structures whose walls nucleate Fe; wüstite remains epitaxial to magnetite.
-
Dominant orientation relationship: (112)ₘₐg // (0001)ₕₑₘ; [110]ₘₐg // [10–10]ₕₑₘ (OR2).
-
Findings elucidate mechanisms relevant to dense regions in industrial pellets and natural ores, supporting microstructure-informed optimization of HyDR reactors.
Reference:
B. Ratzker, M. Ruffino, S. Shankar, D. Raabe, Y. Ma, Acta Materialia 294 (2025) 121174. DOI: 10.1016/j.actamat.2025.121174.