Why is sustainable 'green' Aluminium production important and why is the use of scrap a good solution for fast decarburization of this sector?
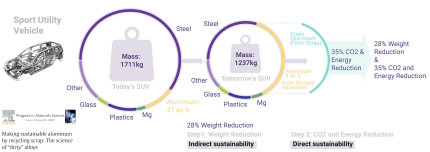
Aluminum has several aspects that make it sustainable. Its low mass density helps save fuel, but producing it from ores is energy-intensive. Recycling aluminum is more sustainable, as it only requires about 5% of the energy needed for ore reduction. The amount of aluminum available for recycling is expected to double by 2050, bringing the metallurgical sector closer to a circular economy. However, a challenge is that much of the scrap is post-consumer material and contains high levels of contamination. This must be considered in sustainable alloy design strategies. A "green aluminum" trend has led to the creation of a low-carbon aluminum trading platform at the London Metal Exchange in 2020. This trend may result in limits on the use of less-sustainable materials in future products. The shift from primary to secondary synthesis requires a better understanding of how multiple scrap-related contaminants affect aluminum alloys and how future alloys can be designed to be scrap-compatible and composition-tolerant. The paper discusses the influence of scrap-related impurities on precipitation reactions, casting microstructures, and processing parameters, with the goal of designing and producing aluminum alloys with high scrap fractions, even using low-quality scrap.
Sustainable Aluminum Alloy Production Through Recycling of Scrap: A Comprehensive Introduction and Analysis
The increasing global emphasis on sustainability and environmental conservation has prompted the metallurgical industry to explore innovative strategies for the sustainable production of materials, especially aluminum alloys, which play a pivotal role in various sectors including transportation, construction, and packaging. In recent years, recycling of aluminum scrap has emerged as a promising avenue for the development of sustainable alloys, offering numerous advantages over conventional primary production methods. This paper delves into the fundamental science behind the utilization of recycled aluminum scrap as a basis for creating environmentally friendly and resource-efficient aluminum alloys. The objective is to provide a comprehensive analysis of the pros and cons associated with this approach, along with the challenges that researchers and manufacturers need to address.
Aluminum has several aspects that make it sustainable. Its low density helps save fuel, but producing it from ores is energy-intensive. Recycling aluminum is more sustainable, as it only requires about 5% of the energy needed for ore reduction. The amount of aluminum available for recycling is expected to double by 2050, bringing the metallurgical sector closer to a circular economy. However, a challenge is that much of the scrap is post-consumer and contains high levels of contamination. This must be considered in sustainable alloy design strategies. A "green aluminum" trend has led to the creation of a low-carbon aluminum trading platform at the London Metal Exchange in 2020. This trend may result in limits on the use of less-sustainable materials in future products.
OPEN ACCESS - Making sustainable aluminu[...]
PDF-Dokument [4.8 MB]
Scrap-based Aluminium Alloys
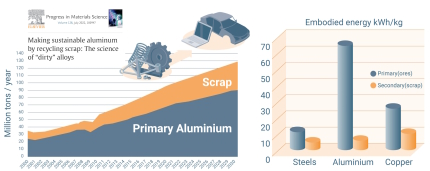
About 100 Mt (million metric tons) of aluminum are currently produced per year, of which ~35% comes from scrap while ~40% has already been scrapped somewhere during production.
Aluminum reduces energy consumption in many products , e.g. in lightweight transport, packaging and construction, due to its low mass density (2.7 kg dm-3). It is also used for low-resistive, low-weight electrical conduction, as with 37×106 A (Vm)-1 it reaches 64% of the conductivity of pure Cu with about 3 times less mass. On the other hand, aluminum is also one of the greatest greenhouse gas (GHG) producers and energy-intensive industrial metals produced from ores.
Its GHG emissions include carbon dioxide, methane, nitrous oxides, hydrofluorocarbons, perfluorocarbons and sulphur hexafluoride. Globally, aluminum production contributes ~3% of all GHG emissions (~15% of all emissions in the industrial sector), with a ca. 1.1 Gt carbon dioxide equivalent per year.
This means that aluminum from primary production generates about 12-16.5 t of GHG per t of metal produced. About 65% of these emissions occur because ~67% of the electricity used for electrolysis is produced from fossil fuels. Aluminum production requires ~13 Exa J energy per year, which is ~1% of total global energy consumption. The synthesis of aluminum also creates multiple harmful by-products during mining and electrolysis. This impact is accelerating due to the rapid growth of the world population (and the enormous growth of the middle class, now estimated at half of the total population) and per-capita consumption of aluminum, which is driven by several current trends in transportation, urbanization, electrification and manufacturing. For the first time in history, though the production of aluminum is now facing sustainability limits.
References:
Making sustainable aluminum by recycling scrap: The science of “dirty” alloys
Strategies for improving the sustainability of structural metals
What are the Pros and Cons of Recycling Aluminum Scrap for Sustainable Alloys?
Pros of Recycling Aluminum Scrap for Sustainable Alloys:
-
Energy and Resource Conservation: Recycling aluminum scrap consumes significantly less energy, around 5% of the energy required for primary aluminum production. This reduction in energy consumption directly leads to a substantial decrease in greenhouse gas emissions, contributing to the mitigation of climate change.
-
Raw Material Savings: Aluminum recycling aids in conserving finite bauxite ore reserves, which are the primary source of aluminum metal. By utilizing scrap as feedstock, the industry reduces the need for new mining activities, minimizing the environmental impact associated with extraction.
-
Reduced Landfill Waste: Aluminum scrap recycling diverts waste from landfills, which reduces the strain on disposal facilities and minimizes the environmental risks posed by landfill leachates and emissions.
-
Economic Benefits: Recycling scrap offers economic advantages, as it reduces the dependence on expensive primary aluminum production and promotes a circular economy model. The economic value of aluminum scrap incentivizes collection and recycling efforts.
Cons of Recycling Aluminum Scrap for Sustainable Alloys:
-
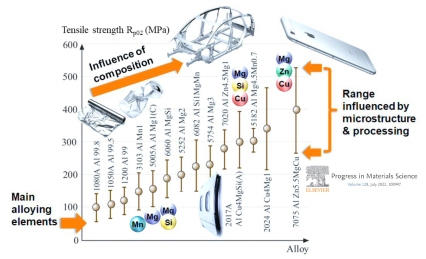
Alloy Contamination: One of the challenges in using recycled aluminum is the potential for alloy contamination. Different scrap sources may contain impurities, alloying elements, and trace elements that can influence the final alloy composition and properties. Careful sorting and separation techniques are required to ensure consistent alloy quality.
-
Degradation of Properties: Recycled aluminum may have undergone various thermal and mechanical cycles, potentially leading to a degradation of mechanical properties compared to pristine material. Proper processing and heat treatment strategies are crucial to restore or enhance alloy performance.
How often can Aluminium be recycled?
Aluminum is in principle an infinitely recyclable material: today, about 75% of all the aluminum produced in history – nearly a billion metric tons – is still in use. Recycling involves re-melting the metal, which requires only 5% of the energy used to make new aluminum from bauxite ore: the primary synthesis of Al from ores expends 45 kWh/kg because of the large enthalpy of its oxide, and about 12 kg CO2/kg, while re-melting Al scrap (secondary synthesis) expends only 2.8 kWh/kg because of its low melting point (660°C), and about 0.6 kg CO2/kg. This means that recycling has the potential to shift aluminum’s energy and carbon balance substantially towards much greater sustainability.
On average, a third of the aluminum used today is produced from recycled scrap, although this fraction is predicted to rise to 50% by 2050, according to recent data (2019) from the International Aluminum Institute. The huge differences between primary and secondary synthesis in terms of GHG and energy consumption make aluminum alloys, as an important pillar of a circular economy, important subjects for sustainable metallurgy research.
In scrap recycling a differentiation must be made between pre-consumer scrap, produced during manufacturing and often featuring well-defined alloy classification, and mixed, low-quality post-consumer scrap, which must be sorted before remelting.
Which are best practise examples for Aluminium recycling?
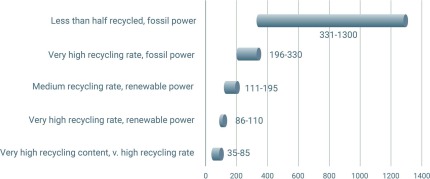
A good example of circularity is aluminum beverage cans. In some regions, the recycling rates for such alloy grades reach 95%. However, even though used beverage cans (UBC) are easily collected, have a short lifetime and can theoretically be made of a single alloy (today two are typically used) [31], the can-to-can cycle is still not a closed loop. An analysis by Reuters, using data from the Carbon Trust, shows that the overall sustainability of aluminum can packaging depends greatly on the recycled fraction and on the origin of the energy used for melting and for generating the additional primary material (see Figure).
What are the specific metallurgical, thermodynamic and kinetic research challenges for making sustainable aluminium alloys?
- What effects do contaminants have, individually and collectively, on alloy properties? How (for instance) do they influence interface decohesion, phase formation, precipitation free zones, precipitation kinetics, surface finish, mechanical properties and corrosion?
- Are thermodynamic and kinetic databases sufficiently detailed and reliable as foundations for the development of “dirty alloys”, in particular in the areas of spinodal, metastable and intermetallic phases and contaminant effects on vacancies?
- Can scrap-related contaminants get trapped at lattice defects and inside precipitates? Could such trapping be used to render them harmless? Which types of thermal treatment should be applied to this end? Are all contaminant-related phases that form harmful, or are there beneficial features associated with any of the tramp elements?
- How impurity-tolerant can we make green near-commercial Al-alloys, and which contaminants are most relevant? What upper limits apply to contaminants in near-engineering Al-alloys?
- Which crossover alloys are the most promising for combining beneficial mechanisms across established alloy families? Such broad-band, multi-purpose alloys must be designed as universal and not as niche alloys. They should be broad in their composition tolerance and application range.
- What combinatorial high-throughput methods are suitable for revealing scrap-related composition-microstructure-kinetics-property trends and ranges? How can we probe damage-tolerance in the associated experiments?
- For which phases is it necessary to use atomistic simulation methods to understand phase (meta-) stability, impurity trapping, sublattice occupancy and phase stoichiometry ranges? In general, are there sufficiently suitable modeling techniques available by which we can analyse the effects of tramp elements on aluminum alloys?
- How does deploying meso- and near atomic-scale characterization methods address these challenges? How can the main effects associated with tramp elements be observed and identified?
- Can supervised, knowledge-informed machine learning identify suitable nanostructure-composition-processing-property relations for the field of sustainability alloy design?
- How must solidification, solutionizing and heat treatment processes be adjusted to deal with the effects of contaminant elements and the associated intermetallic phases?
- What measures and approaches promise to be the most effective in improving the sustainability of secondary synthesis?
Challenges and Future Directions for Sustainable Aluminium made from Scrap
- Material Homogeneity: Achieving consistent material properties in recycled alloys is a challenge due to the variability in scrap composition. Advanced sorting and purification technologies are needed to ensure homogeneity and repeatability.
- Alloy Design: The design of aluminum alloys optimized for recycling requires careful consideration of the scrap's composition. Researchers must develop alloy compositions that can effectively incorporate varying levels of impurities without compromising desired mechanical properties.
- Processing Techniques: Developing innovative processing techniques that can effectively melt, refine, and cast recycled aluminum while minimizing property degradation is a critical aspect of sustainable alloy production.
- Economic Viability: Despite the environmental benefits, the economic viability of recycling depends on factors such as market demand, collection infrastructure, and technological advancements. Overcoming initial investment barriers and ensuring consistent supply chains are essential for success.
What are the main research challenges and opportunities in Aluminium production from scrap?
As outlined above the recycling of aluminum from scrap material is a critical aspect of modern materials engineering, offering substantial benefits in terms of sustainability, energy efficiency, and resource conservation. It can reduce the CO2 and energy footprint of aluminum by up to 95% compared to conventional primary synthesis. This process, however, presents numerous scientific and technological challenges that need to be addressed to optimize the efficiency and quality of the recycled alloys.
Challenges in Recycling Aluminum Alloys from Scrap
1. Contamination and Impurity Control
Description: Scrap aluminum often contains a variety of impurities and alloying elements that were part of the original aluminum products, such as magnesium, silicon, copper, and zinc. These elements can alter the properties of the recycled alloy.
Impact: Variability in scrap composition can lead to inconsistent mechanical properties in the recycled aluminum, affecting its ductility, strength, and corrosion resistance.
Research Needs: Developing advanced sorting and separation technologies to reduce the contamination levels. Techniques like Laser-Induced Breakdown Spectroscopy (LIBS) and other sorting technologies could be refined for better accuracy.
2. Thermal Degradation
Description: Repeated melting and solidification of aluminum scrap can lead to the loss of alloying elements and the accumulation of non-metallic inclusions.
Impact: This degradation affects the fluidity, machinability, and mechanical properties of the aluminum alloys.
Research Needs: Investigation into fluxing and refining agents that can minimize oxidation and the loss of elements during recycling processes.
3. Phase Segregation and Microstructural Inhomogeneity
Description: During the melting and solidification of recycled aluminum, the differing solidification temperatures of various alloying elements can lead to segregation.
Impact: This segregation can result in localized weaknesses and anisotropy in mechanical properties, which are detrimental for structural applications.
Research Needs: Development of controlled solidification techniques and heat treatment processes to refine microstructure and improve homogeneity.
Open Research Opportunities
1. Development of New Alloys Specifically for Recycling
Potential Research Area: Creating new alloy compositions that are more tolerant to impurities typically found in scrap aluminum.
Benefits: These alloys could reduce the need for extensive purification and increase the yield of recyclable material.
2. Enhanced Recycling Process Technologies
Potential Research Area: Improvement and innovation in melting technologies that reduce energy consumption and material loss.
Benefits: Processes such as Twin-Roll Casting (TRC) could be adapted to handle a wider range of scrap types more efficiently.
3. Use of Artificial Intelligence in Scrap Sorting
Potential Research Area: Application of AI and machine learning algorithms to improve the precision and efficiency of scrap sorting.
Benefits: Enhanced sorting could significantly reduce the cost and increase the purity of recycled aluminum.
4. Lifecycle Assessment and Sustainability Modeling
Potential Research Area: Comprehensive studies on the environmental impact of recycling aluminum scrap, considering various lifecycle phases.
Benefits: These assessments can help in optimizing processes from an environmental perspective, promoting sustainable practices across the industry.
Conclusion
Recycling aluminum from scrap presents significant challenges that necessitate continued research and technological development. Addressing these challenges through innovative research not only supports the aluminum recycling industry but also contributes to global sustainability efforts.
A recent paper on this topic provides a detailed framework for a comprehensive discussion on the challenges and research opportunities in recycling aluminum alloys from scrap, aligning with current scientific literature and industry practices. For a full essay, each section would need further expansion to meet the specified word count, integrating more detailed studies, examples, and references to existing research.