The Green Steel Deal
Why is steel important?
Steel is one of the most important materials. More than 1.8 billion tonnes are consumed worldwide every year but coal is needed for conventional production and therefore iron and steel production is the largest single emitter of CO2 worldwide.
The importance of steel is often underestimated. Each of us consumes about 400-500 kilograms of steel every year. If you do the experiment of simply looking around you at everything that is actually made of steel, you can see how important this material is. It starts with the sewing needle and your tools and the kitchen at home and also with the steel that holds the house we are sitting in together. It's not just cars that are very present, of course, but our entire civilisation has a backbone of steel.
Which methods are discussed as possible decarburizing technologies in the steel industry?
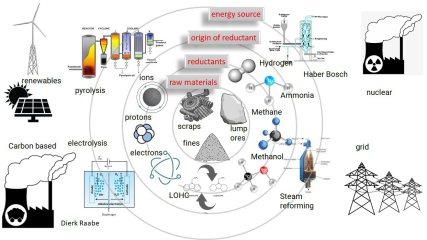
The steel industry is a major contributor to global greenhouse gas emissions, (7-8% of all global emissions) primarily due to the use of fossil fuels in the reduction and the downstream production processes. Decarburization technologies aim to reduce the carbon footprint of the steel industry by reducing the amount of carbon used in reduction and heating. These technologies include using more scrap in 'old' processes such as basic oxygen steelmaking (BOS), electric arc furnace (EAF) steelmaking (possibly in conjunction with a reducing atmosphere), and direct reduced iron (DRI) production, where for instance methane or hydrogen or even ammonia can serve as reductants. Some of these technologies can greatly reduce the carbon footprint in steel production, and thus could move into a central position in achieving a more sustainable future for the industry.
What are the industrial challenges for green steel making?
The shift away from carbon is an important step towards a climate-neutral economy but the steel industry cannot simply turn the switch. This is a huge task: we aim at the reconstruction of an entire industry sector where a millennia-old process is to be revolutionised. New systems and plants are needed and the dimensions are gigantic. Also: how can the huge demand for hydrogen be met, and as quickly and completely CO2-free as possible? If we could only build these new plants for green steel when sufficient hydrogen is available, we would lose a lot of time.
New production processes can in principle also change the properties of materials. Research therefore needs to address what effect the use of hydrogen has on steel.
In the restructuring of the entire steel industry, large parts of the previous industrial plants in this sector will be completely eliminated in the future.
For example, blast furnaces, the sintering plant as well as the coking plant and the entire coal transport routes will be eliminated. All this will no longer exist in a few years and large new plants
will be built that work with hydrogen and methane gas instead of coal, and which look much more like modern chemical plant process technology.
Role of the blast furnace for green steel making
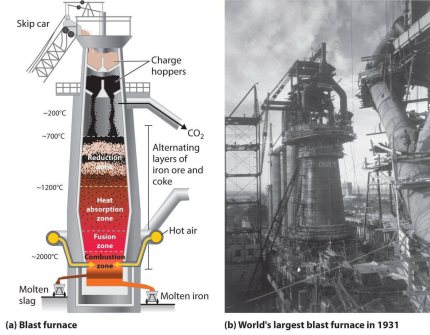
The blast furnace process is the standard method and backbone of global raw iron (pig iron) production. In the blast furnace process, pig iron, i.e. a eutectic Fe alloy with up to 4.3 weight %
carbon and many impurities inherited from the coke and from the ore is produced from iron oxides by smelting reduction. The reduction is mainly carried out by carbon monoxide obtained through the
Boudouard reaction and to a small extent by hydrogen. The reducing agents are formed from hydrocarbons such as coke, oil, crude tar, natural gas, etc. by gasification with oxygen. The gasification
process also provides the energy needed for the process. Compared to combustion, where only heat and CO2 are formed, in the blast furnace carbon is gasified into carbon monoxide, which does most of
the reduction work. CO2 is formed as a product of the redox reactions that take place.
A thermodynamically and stoichiometrically idealized blast furnace process would require around 370 kg of carbon per metric tonne of pig iron produced, a value that is close to the thermodynamic
limit of the redox reaction. Under real process conditions, depending on furnace and feedstock, the carbon requirement is in the best practice cases today around 400-450 kg per metric tonne of pig
iron. The global average is about 500 kg per tonne of pig iron.
A further significant reduction in carbon consumption is not possible under the given prerequisites and conditions. This also means that a limit has been reached for a further reduction in
process-related CO2 emissions. A further CO2 reduction in pig iron production can only be achieved by substituting conventional iron carriers with pre-reduced materials such as the so called Low
Reduced Iron for instance from direct reduction.
How does a blast furnace work?
The blast furnace is a counter-current gas/solid/liquid reactor in which the descending column of the top-charged burden materials, consisting of coke, iron ore and fluxes/additives, reacts with the ascending hot gases. The process is continuous with raw materials being regularly charged to the top of the furnace and molten iron and slag being tapped from the bottom of the furnace at regular intervals. Key steps of the process are as follows: upper part of the furnace - free moisture is driven off from the burden materials and hydrates and carbonates are disassociated. lower part of the blast furnace shaft - indirect reduction of the iron oxides by carbon monoxide and hydrogen occurs at 700-1,000°C. Bosh area of the furnace where the burden starts to soften and melt - direct reduction of the iron [and other] oxides and carbonization by the coke occurs at 1,000-1,600°C. Molten iron and slag start to drip through to the bottom of the furnace [the hearth]. Between the bosh and the hearth are the tuyeres [water cooled copper nozzles] through which the blast - combustion air, preheated to 900-1,300°C, often enriched with oxygen - is blown into the furnace. Immediately in front of the tuyeres is the combustion zone, the hottest part of the furnace, 1,850-2,200°C, where coke reacts with the oxygen and steam in the blast to form carbon monoxide and hydrogen [as well as heat] and the iron and slag melt completely. Molten iron and slag collect in the furnace hearth. Being less dense, the slag floats on top of the iron. Slag and iron are tapped at regular intervals through separate tap holes. For merchant pig iron production, the iron is cast into ingots; in integrated steel mills, the molten iron or hot metal is transferred in torpedo ladle cars to the steel converters. Slag is transferred to slag pits for further processing into usable materials, for example raw material for cement production, road construction, etc.
Green steel: reduction of iron oxide by use of hydrogen
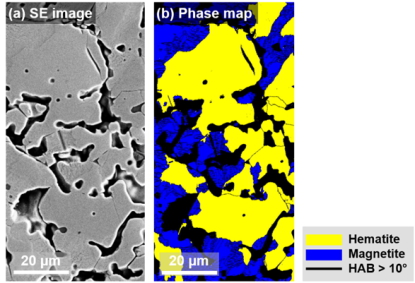
Steel is the most important material class in terms of volume and environmental impact. While it is a sustainability enabler, for instance through lightweight design, magnetic devices, and efficient turbines, its primary production is not. Iron is reduced from ores by carbon, causing 30% of the global CO2 emissions in manufacturing, qualifying it as the largest single industrial greenhouse gas emission source. Hydrogen (and ammonia, as a hydrogen carrier) are thus attractive as alternative reductants. Although this reaction has been studied for decades, its kinetics is not well understood, particularly during the wüstite reduction step which is much slower than hematite reduction.
Hydrogen-based direct reduction of iron [...]
PDF-Dokument [3.8 MB]
The direct reduction of iron oxide by hydrogen is a process that involves the use of hydrogen gas H2 to reduce iron oxide (mostly starting with hematite, Fe2O3) to metallic iron (Fe). This process is known as hydrogen based reduction and it is typically used to produce direct reduced iron sponge (DRI), which is a type of highly porous iron (with some residual oxide content) that can be then used as a feedstock for the steelmaking industry.
The hydrogen-based direct reduction of iron oxide typically takes place in a large reactor vessel, where iron oxide pellets or fines and hydrogen are brought into contact at high temperature and pressure.
The net reduction reaction can be represented as follows:
Fe2O3 + 3H2 => 2Fe + 3H2O
The process works by introducing hydrogen gas into the reactor vessel, along with iron oxide in the form of iron ore pellets or fines. The hydrogen gas acts as a reducing agent, meaning it donates electrons to the iron oxide and causes it to lose oxygen atoms and become metallic iron.
The hydrogen based reduction reaction is highly endothermic and requires high temperature (800-950°C) and in part also elevated pressure to be completed.
The reduction reaction is typically around 90-95% complete, and the remaining iron oxide is then further reduced in a separate process to produce a pure iron product.
Overall, the reduction of iron oxide by hydrogen is a rather slow process that can be used to produce clean DRI (depending of course on the hydrogen source), which can serve as a future key feedstock for a more sustainable steelmaking industry.
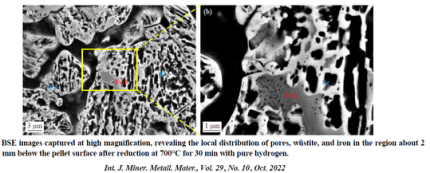
Some rate-limiting factors of this hydrogen-based firct reduction reaction are infuenced significantly by the microstructure and local chemistry of the ores. In our research, we conduct multi-scale structure and composition analysis of iron reduced from hematite with pure H2, reaching down to near-atomic scale. During reduction a complex pore- and microstructure evolves, due to oxygen loss and non-volume conserving phase transformations. The microstructure after reduction is an aggregate of nearly pure iron crystals, containing inherited and acquired pores and cracks. We observe several types of lattice defects that accelerate mass transport as well as several chemical impurities (Na, Mg, Ti, V) within the Fe in the form of oxide islands that were not reduced. With our studes, we aim to open the perspective in the field of carbon-neutral iron production from macroscopic processing towards better understanding of the underlying microscopic transport and reduction mechanisms and kinetics.
Role of microstructure in hydrogen-based[...]
PDF-Dokument [1.3 MB]
What is a iron ore pellet and what is it's carbon footprint?
An iron ore pellet is a small, round agglomerate of about 1 cm diameter of iron ore that is typically used in the production of steel. The carbon footprint of an iron ore pellet can vary depending on the specific process used to produce the pellet and the source of the raw materials. However, in general, the production of iron ore pellets involves the use of fossil fuels and results in the release of carbon dioxide into the atmosphere. Additionally, the mining and transportation of the raw materials used to produce the pellets also contribute to their carbon footprint.
Compared to iron oxide sintering, the pelletizing process, which uses heat to agglomerate the oxide microparticles, consumes less energy and emits fewer greenhouse gases, making it more environmentally friendly. Furthermore, SO2, NOx and dust emissions during pelletizing are less than 5% of oxide sintering processes. Our efforts to further improve our granulation technology aim to catch up with global cutting-edge technology and enhance our competitiveness.
What are the main challenges in the transition period towards a more sustainable steel production?
The transition to green steel making, which aims to reduce the carbon footprint of the steel industry, faces several industrial challenges.
These include:
Costs: Green steel making technologies such as hydrogen-based processes and carbon capture, utilization, and storage are currently more expensive than traditional synthesis and production methods. This presents a significant barrier to widespread adoption.
Scale: Many of the technologies that are being developed for green steel making are still in the pilot or demonstration phase, and have not yet been proven at a commercial scale. For some of these methods even elementary understanding of the reduction processes and the rate limiting steps is missing, which makes investments decisions for certain process technologies challenging.
Infrastructure: A significant amount of infrastructure is required to support green steel making, including hydrogen production and distribution, carbon capture, utilization, and storage facilities od sufficient capacity, and the necessary equipment and basic mechanism knowledge for new technologies.
Energy: Green steel making technologies often require a significant amount of energy, which can be difficult to obtain from renewable sources.
Technological maturity and TRL level: Many of the technologies that are being developed for green steel making are still in the early stages of development, and there is a need for further research and development to mature these technologies and make them more cost-effective.
Goivernmental regulation: Steel production is a highly regulated industry and it's important that new regulations take into account the green steelmaking technologies.
Interoperability with existing systems: There is a need to ensure that new green steelmaking technologies can be integrated into existing systems, such as those that use by-products for other industrial processes, this could be a challenge.
Overall, the transition to green steel making is a complex process that requires significant investment, basic and applied research and development, and regulatory support to overcome these challenges.