Green Alumunium and the Science of 'Dirty Alloys'
What is aluminium used for?
Aluminum, also spelled aluminium, is a lightweight, corrosion-resistant metal that has a wide variety of uses.
Some of the most common uses of aluminum include:
- Transportation: Aluminum is used to make cars, trucks, trains, and airplanes. It is lightweight, which makes vehicles more fuel-efficient, and it is also resistant to corrosion, which extends the life of the vehicle.
- Construction: Aluminum is used in the construction industry to make doors, windows, siding, roofing, and other building components. It is strong and durable, but also lightweight, which makes it easy to work with and transport.
- Packaging: Aluminum is used to make cans, foils, and other forms of packaging. It is lightweight, which makes it easy to transport and store, and it is also an excellent barrier against light, air, and moisture, which helps to preserve the contents of the packaging.
- Electrical equipment: Aluminum is used to make electrical conductors and transformers. It is a good conductor of electricity and it is resistant to corrosion, which makes it well-suited for use in electrical equipment.
- Consumer products: Aluminum is used in a wide variety of consumer products, such as bicycles, lawn furniture, and kitchen utensils. It is lightweight, which makes it easy to use and transport, and it is also resistant to corrosion, which makes it durable.
- Industrial applications: Aluminum is used in a variety of industrial applications such as heat exchangers, fuel and chemical tanks, as well as in the production of aluminum alloys.
- Aerospace: Aluminum alloys are used in aerospace industry to make airplane parts, such as the fuselage, wings and even the engines.
Overall, aluminum's versatility, durability and resistance to corrosion make it an essential material in many industries.
What is red mud?
Red mud is a byproduct of the industrial process of extracting alumina (aluminum oxide) from bauxite ore, which is used to make aluminum. It is called "red mud" because of its reddish color, which comes from the iron oxide in bauxite ore. Red mud is a highly alkaline waste material that can contains toxic heavy metals and even radioactive elements. It is considered a hazardous waste and must be properly disposed of.
Motivation for making aluminum alloys from scrap
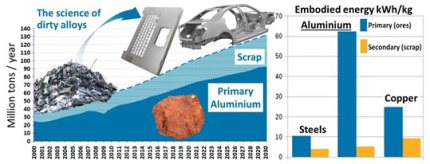
More than 100 Mt (million metric tons) of aluminum are currently produced per year, of which ~35% comes from scrap while ~40% has already been scrapped during manufacturing. Aluminum is a material with two sides when it comes to sustainability. On the one hand, it reduces energy consumption in multiple products and processes, e.g. in lightweight transport, packaging and construction, due to its low mass density (2.7 kg dm-3). It is also increasingly used for low-resistive, low-weight electrical conduction, as with 37×106 A (Vm)^-1 it reaches 64% of the conductivity of pure Cu with about 3 times less mass. On the other hand, aluminum is also one of the greatest greenhouse gas (GHG) producers and energy-intensive industrial metals produced from ores . Its GHG emissions include carbon dioxide, methane, nitrous oxides, hydrofluorocarbons, perfluorocarbons and sulphur hexafluoride. Globally, aluminum production contributes ~3% of all GHG emissions (~15% of all emissions in the industrial sector), with a ca. 1.1 Gt carbon dioxide equivalent per year. This means that aluminum from primary production generates about 12-16.5 t of GHG per t of metal produced.
About 65% of these emissions occur because ~67% of the electricity used for electrolysis is produced from fossil fuels. Aluminum production requires ~13 Exa J energy per year, which is ~1% of total global energy consumption. The synthesis of aluminum also creates multiple harmful by-products during mining and electrolysis. This impact is accelerating due to the rapid growth of the world population (and the enormous growth of the middle class, now estimated at half of the total population) and per-capita consumption of aluminum, which is driven by several current trends in transportation, urbanization, electrification and manufacturing. For the first time in history, though the production of aluminum is now facing sustainability limits.
Aluminum alloys made entirely from scrap can reduce energy consumption and GHG emissions by >90% compared to alloys made by primary synthesis. Advanced alloy design aims at improving and developing alloys via built-in recyclability, for maximum scrap compatibility and use [15]. On average the values for the embodied energy for primary aluminum production range between 53 and 65 kWh/kg (190 – 235 MJ / kg), depending on slightly different approximations and year.
Why make aluminium from scrap?
There are several reasons why using scrap aluminum to produce new aluminum is beneficial:
Energy savings: Recycling aluminum requires only 5% of the energy needed to produce new aluminum from raw materials. This means that recycling aluminum can significantly reduce the energy needed to produce new aluminum, which in turn reduces carbon emissions and saves on energy costs.
Resource conservation: Recycling aluminum helps to conserve natural resources by reducing the need to extract new bauxite (aluminum ore) from the earth. Bauxite mining can have negative environmental impacts, such as deforestation, habitat destruction, and water pollution.
Cost savings: Using scrap aluminum to produce new aluminum can be more cost-effective than producing new aluminum from raw materials. This is because the cost of the raw materials and energy needed to produce new aluminum can be high, whereas scrap aluminum is often cheaper and readily available.
Reduced Waste: Recycling aluminum reduces the amount of waste that goes to landfills, which can help to reduce pollution and conserve space.
Increased demand: The demand for aluminum scrap is increasing as more and more companies are realizing the benefits of recycling aluminum. This increased demand can lead to more jobs in the recycling industry and can help to create a more sustainable economy.
Overall, recycling aluminum is an environmentally friendly, cost-effective and resource-efficient way to produce new aluminum, which is why it is beneficial to make aluminum from scrap.
Aluminium has two faces when it comes to sustainability
Aluminium has two sides when it comes to sustainability. It helps saving fuel due to its low mass density (called indirect sustainability), yet, it is a very carbon-intensive metal to produce from ores (called direct sustainability). Recycling shifts this balance towards higher sustainability since even when using hydro power for primary synthesis, the energy needed for melting aluminium from scraps is only about 5% of that consumed for reducing ores.
What is the role of scrap for green Aluminium?
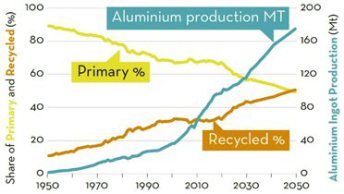
Recycling shifts this balance towards higher sustainability since even when using hydro power for primary synthesis, the energy needed for melting aluminium from scraps is only about 5% of that consumed for reducing ores. The amount of aluminium available for recycling is estimated to double by 2050, offering opportunities to bring the metallurgical sector closer to the global goal of a circular economy. The ‘green aluminium’ trend has even triggered a new trading platform in 2020 for low-carbon aluminium at the London Metal Exchange. A consequence might be that less sustainable materials might experience limitations for use in future products.
Aluminum is in principle an infinitely recyclable material: today, about 75% of all the aluminum produced in history – nearly a billion metric tons – is still in use. Recycling involves re-melting the metal, which requires only 5% of the energy used to make new aluminum from bauxite ore: the primary synthesis of Al from ores expends 45 kWh/kg because of the large enthalpy of its oxide, and about 12 kg CO2/kg, while re-melting Al scrap (secondary synthesis) expends only 2.8 kWh/kg because of its low melting point (660°C), and about 0.6 kg CO2/kg. This means that recycling has the potential to shift aluminum’s energy and carbon balance substantially towards much greater sustainability.
On average, a third of the aluminum used today is produced from recycled scrap , although this fraction is predicted to rise to 50% by 2050, according to recent data (2019) from the International Aluminum Institute. The huge differences between primary and secondary synthesis in terms of GHG and energy consumption make aluminum alloys, as an important pillar of a circular economy, important subjects for sustainable metallurgy research.
Today aluminum recycling is conducted primarily within established alloy classes, and only small scrap tonnages are exchanged among compositionally dissimilar alloy groups.
The recycling rates for aluminum are generally rather high, but they differ substantially by product, alloy and region, ranging from as low as 20% to as high as 80% for some packaging products.
The quality, abundance and flow of scrap in this field vary greatly, as do the target alloys and their composition requirements. They range from highly contaminated and mixed low-price scraps (also referred to as old scrap or post-consumer scrap) and lower-purity-sensitive commodity alloys to well-defined high-quality secondary material (retrieved from closed-loop industry processes where little further improvement can be achieved) and very impurity-sensitive high-performance alloys with little tolerance for tramp elements. It is this variety in the scrap landscape on the one hand, and the great diversity in the types of aluminum alloy and the resulting products on the other, which set the stage for our investigation of the role of scrap-usage in aluminum alloys and their potential recycling-friendly design.
What kind of research is required for green Aluminium?
Identifying pathways towards Sustainability Alloy Design requires to understand and utilize how multiple scrap-related contaminant elements act on aluminium alloys, coining the term ‘Science of Dirty Alloys’, and how alloys can be upfront designed to become ‘scrap-compatible’, for maximized use of scrap, a property that equips these materials with the ‘Gene of Recyclability’. This paper addresses the metallurgical fundamentals behind the aim to develop, process and optimize aluminium alloys for performance, using higher scrap fractions. We aim at developing design guidelines for alloys with more built-in recyclability, which targets a shift from primary synthesis (reducing ores) to secondary synthesis (melting scraps). Exploring suited pathways towards Sustainability Alloy Design requires to identify, understand and exploit the underlying metallurgical mechanisms behind the use of high scrap fractions. This includes topics such as the influence of scrap-related impurity ingress on thermodynamics and kinetics of precipitation reactions as well as their mechanical and electrochemical effects; precipitation free zones around grain boundaries; casting microstructures; adjustment of processing parameters, as well as the resulting mechanical, functional and chemical properties. For this we also review advanced experimental probing methods to identify the partitioning of tramp elements between the lattice defects, the precipitate phases and the matrix and the associated effects on precipitation kinetics and thermodynamics and mechanical response. The goal of the article is to guide the design and production of aluminium alloys from highest possible scrap fractions, low quality scraps and scrap types with only few matching target alloys they can serve in when recycled. Discovering the underlying foundations of this field can guide the direction for the development of a generation of more sustainable metallic materials. This can be a fundamental contribution not only to the metallurgical sciences but also to a more sustainable society and a circular economy. Translation of such ambitions into successful, safe and high performance products that can meet highest quality standards requires deep understanding of the basic science behind sustainable metallurgy and recycled ‘green’ alloys, establishing a new field of the Science of Dirty Alloys.
https://www.sciencedirect.com/science/article/pii/S0079642522000287
Sustainable aluminum by recycling scrap [...]
PDF-Dokument [4.1 MB]
There are several basic research questions with respect to the metallurgy of these recycled aluminum
alloys. One set of questions concerns the number and quantities of scrap-related tramp elements that can be tolerated in existing high-performance Al-alloys, and what can be done to make them
more impurity-tolerant while retaining comparable properties.
The extensive use of scrap for secondary synthesis of aluminum alloys from in-production, post-production and post-consumer sources turns the underlying phase diagrams of these alloys into multi-component equilibria scenarios: while conventional phase diagrams for aluminum alloys reside in low-dimensional composition corners with one or two prevalent alloying elements, the phase diagrams required to design future scrap-tolerant alloys may have to juggle up to 15 composition axes. This is particularly important for aluminum alloys, due to their low solubility for most scrap-related tramp elements.
Another set of challenges lies in whether new alloy design strategies might target a compromise between higher scrap usage on the one hand, and modestly reduced properties that satisfy the requirements of a broader range of commodity products on the other. This trade-off could be quantified by balancing the reduction in energy use and GHG production through higher scrap use with performance loss and the associated sustainability reduction when the material is in service. Other questions concern the design of new types of alloy, the so-called crossover or uni-alloys, with extensive chemical overlap between separate alloy classes. If sufficient performance is achieved, such materials would be more recycling-friendly from the outset. Another avenue would be to develop compositionally lean alloys, which realize properties less through fine-tuning of composition than through processing and microstructure tuning. This allows the decoupling of properties from compositions and permits compositionally simple alloys to be more readily recycled and mixed.