Hydrogen-based co-reduction of multicomponent oxides
Motivation for this Project
The decarbonization of metallurgical processes is critical for achieving global sustainability goals, with hydrogen-based direct reduction (HyDR) emerging as a key technology to replace fossil fuel-based methods. This short review examines the role of oxide precursor states—mechanically mixed compacted powders versus chemically mixed pre-sintered compounds—in the HyDR of multicomponent oxides targeting equiatomic Co-Fe-Mn-Ni alloys. Drawing from recent experimental insights, we highlight how precursor morphology and phase composition influence reduction kinetics, thermodynamics, and resulting microstructures. Thermogravimetric analysis reveals distinct reduction pathways: sequential for compacted powders (onset ~175 °C) and concurrent for pre-sintered samples (onset ~525 °C), both achieving ~80% reduction at 700 °C. Pulverization of pre-sintered precursors decouples kinetic and thermodynamic effects, lowering onset temperatures while maintaining higher stability of solid-solution phases. Thermodynamic modeling via CALPHAD confirms enhanced stability of halite and spinel phases in pre-sintered precursors, leading to delayed reduction and unique microstructural features like residual BCC Fe. These findings underscore the potential for tailored precursor design in one-step sustainable alloy production, integrating reduction, alloying, and sintering.
1. Introduction
The metallurgical sector contributes approximately 40% of industrial greenhouse gas emissions, primarily due to carbon-intensive reduction processes [1,2]. Transitioning to hydrogen as a reductant offers a pathway to carbon-neutral metal production, producing water as the sole byproduct [3–5]. HyDR of iron oxides has been extensively studied [6–8], but extending this to multicomponent systems enables direct synthesis of complex alloys in a single process, bypassing energy-intensive melting and remelting steps [9–11].
A critical factor in HyDR is the initial state of oxide precursors: mechanically mixed (individual oxides blended without atomic-scale mixing) versus chemically mixed (pre-sintered solid solutions). Natural ores often resemble the latter, with low metal content and complex mineralogy [12], while synthetic precursors allow controlled compositions. Recent work on Co3O4-Fe2O3-Mn2O3-NiO mixtures targeting 25Co-25Fe-25Mn-25Ni (at.%) alloys demonstrates profound differences in reduction behavior based on precursor type [13]. Mechanically mixed precursors promote faster kinetics due to high porosity (~26%), while pre-sintered ones form stable halite ((Co,Ni)O) and spinel ((Fe,Mn)3O4) phases, delaying reduction but enabling unique microstructural control.
This review synthesizes key insights from thermogravimetric analysis (TGA), synchrotron X-ray diffraction (HEXRD), microstructural characterization, and thermodynamic modeling. We discuss how precursor states decouple kinetics and thermodynamics, offering strategies for sustainable one-step metallurgy [14].
2. Experimental and Theoretical Approaches
2.1 Precursor Preparation and Reduction
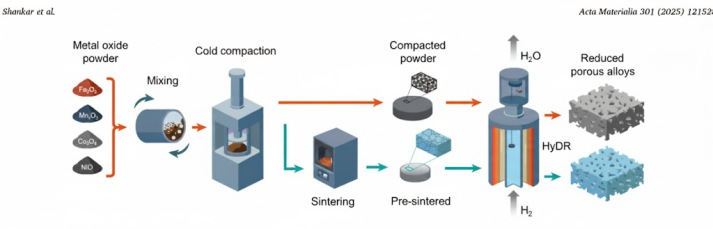
Oxide powders (Co3O4, Fe2O3, Mn2O3, NiO; ≥99% purity) were weighed to equiatomic metal ratios and ball-milled for homogenization. Compacted powders were pressed at ~225 MPa into discs. Pre-sintered
samples were heated to 1100 °C under Ar for 5 h, forming dual-phase microstructures (55 wt.% spinel, 45 wt.% halite). To isolate thermodynamic effects, pre-sintered samples were pulverized via
extended ball-milling (5–10 h).
HyDR was performed in a custom TGA setup [15] under 100% H2 at 10 °C/min to 700 °C, with 1 h isothermal hold. Mass loss reflected oxygen removal, targeting ~80% reduction (full for Co, Fe, Ni; partial for Mn to MnO).
OPEN ACCESS Paper: https://www.sciencedirect.com/science/article/pii/S1359645425008146
Acta 2025 H-based direct reduction multi[...]
PDF-Dokument [21.9 MB]
2.2 Characterization and Modeling
Phases were analyzed via HEXRD at PETRA III (60 keV, λ=0.207 Å) and Rietveld refinement. Microstructures were examined using SEM, EDS, and EBSD. Thermodynamic calculations used Thermo-Calc with
TCOX10 database, predicting Gibbs free energies and phase stability as functions of temperature and oxygen partial pressure (pO2).
Table 1 summarizes initial phase compositions.
**Table 1: Phase Fractions (wt.%) in Initial Precursors (from HEXRD Rietveld Refinement)**
| Precursor Type | Co3O4 | Fe2O3 | Mn2O3 | NiO | Spinel | Halite |
|----------------------|-------|-------|-------|------|--------|--------|
| Compacted Powder | 20.3 ± 1.7 | 26.0 ± 1.7 | 28.2 ± 2.3 | 25.5 ± 1.6 | - | - |
| Pre-Sintered | - | - | - | - | 55.0 ± 1.5 | 45.0 ± 1.3 |
3. Results and Discussion
3.1 Reduction Kinetics and Pathways
TGA revealed stark differences in reduction onset: ~175 °C for compacted powders versus ~525 °C for pre-sintered samples (Fig. 1, analogous to original data). Both reached ~80% reduction after 1 h at 700 °C, but pathways diverged. Compacted powders exhibited three rate peaks, indicating sequential reduction (e.g., Fe2O3 first, per Ellingham stability [16]), while pre-sintered samples showed a single peak with a shoulder, suggesting concurrent halite-spinel reduction.
Pulverization lowered pre-sintered onset to ~175 °C (10 h milling), but retained a delayed major event (~100 °C shift), highlighting thermodynamic dominance. Peak rates were higher for pre-sintered (~0.12 s⁻¹) due to diffusion-limited reactions at elevated temperatures.
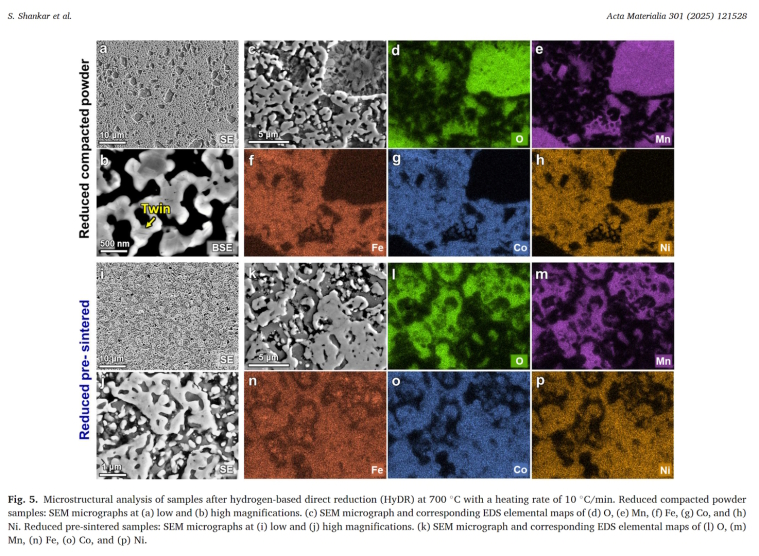
HEXRD confirmed post-reduction phases: FCC (70 wt.%, Co-Fe-Ni solid solution) and MnO (30 wt.%). Pre-sintered samples uniquely contained ~1 wt.% BCC Fe, attributed to local Fe sequestration near MnO interfaces, preventing austenite stabilization by Co/Ni.
3.2 Microstructural Evolution
Initial compacted powders were porous (~26%) with heterogeneous Mn2O3 distribution (0.2–6 µm particles). Pre-sintered samples were dense (~98%), with ~2 µm grains of halite (Co-Ni-rich) and spinel
(Fe-Mn-rich).
Post-HyDR, both exhibited ~15% porosity from oxygen removal and sintering. Compacted samples showed large MnO agglomerates, reflecting initial heterogeneity. Pre-sintered microstructures preserved dual-phase patterns, with finer, uniform MnO and submicron BCC Fe grains surrounded by MnO (EBSD confirmation). Annealing twins in FCC indicated solid-state recrystallization.
3.3 Thermodynamic Insights
CALPHAD modeling showed lower Gibbs energies for mixed phases: (Co,Ni)O and (Fe,Mn)3O4 more stable than individual oxides, explaining delayed reduction. Multicomponent Ellingham diagrams predicted sequential metallization: Ni/Co first, then Fe, with Mn stabilizing residual (Fe,Mn)O.
Phase evolution with pO2 (at 700 °C) indicated halite-spinel coexistence at higher pO2, transitioning to FCC + spinel I, then full FCC at low pO2 (~10⁻³² atm). EDS validated residual Fe in MnO, confirming solid-solution effects hindering complete Fe reduction.
Table 2 compares reduced phase fractions.
Table 2: Phase Fractions (wt.%) in Reduced Samples
| Precursor Type | FCC | MnO | BCC |
|----------------------|-------|-------|-------|
| Compacted Powder | 70 ± 1.0 | 30 ± 0.7 | - |
| Pre-Sintered | 69 ± 1.1 | 30 ± 0.8 | 1 ± 0.2 |
Implications for Sustainable Alloy Design
Precursor state enables control over HyDR: mechanical mixing favors kinetics for rapid processing, while pre-sintering enhances thermodynamic stability for tailored microstructures (e.g., dispersed
oxides for strengthening). This aligns with one-step metallurgy [14], potentially reducing energy use by 50–70% compared to traditional routes.
Challenges include scaling to natural ores and optimizing H2 efficiency. Future work should explore variable compositions and higher temperatures for full Mn reduction.
Conclusions
HyDR of multicomponent oxides is profoundly influenced by precursor state, with compacted powders enabling fast, sequential reduction and pre-sintered compounds yielding stable phases for microstructural control. Decoupling kinetics via pulverization reveals thermodynamic barriers in solid solutions, supported by CALPHAD. These insights pave the way for sustainable, one-step production of complex alloys, reducing emissions and resource intensity in metallurgy.
References
[1] D. Raabe et al., Nature 575 (2019) 64–74.
[2] D. Raabe, Chem. Rev. 123 (2023) 2436–2608.
[3] J.M. Chen, Innovation 2 (2021) 100127.
[4] D. Spreitzer, J. Schenk, Steel Res. Int. 90 (2019) 1900108.
[5] Y. Ma et al., Scr. Mater. 213 (2022) 114571.
[6] W.K. Jozwiak et al., App. Catal. A 326 (2007) 17–27.
[7] Y. Ma et al., Inter. J. Miner. Metall. Mater. 29 (2022) 1901–1907.
[8] H.-Y. Lin et al., Mater. Chem. Phys. 85 (2004) 171–175.
[9] P. Bracconi, L.C. Dufour, J. Phys. Chem. 79 (1975) 2395–2400.
[10] Y. Zhang et al., J. Min. Metall. 49 (2013) 13–20.
[11] C. Kenel et al., Acta Mater. 193 (2020) 51–60.
[12] Q. Dehaine et al., Miner. Eng. 160 (2021) 106656.
[13] S. Shankar et al., Acta Mater. 301 (2025) 121528.
[14] S. Wei et al., Nature 633 (2024) 816–822.
[15] M. Auinger et al., Rev. Sci. Instrum. 84 (2013) 085108.