Steels: The strongest ductile bulk materials available
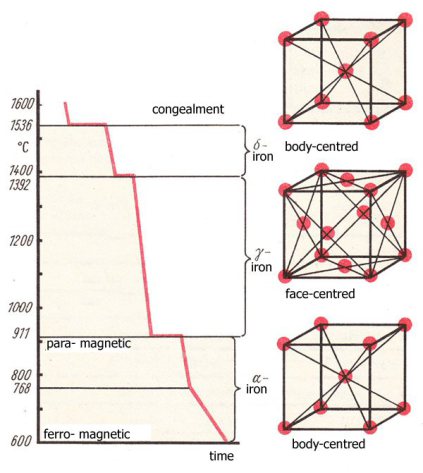
Iron and its allotropes
-
- Allotropes of ferrite include:
- α-iron (Alpha ferrite)
- δ-iron (Delta ferrite)
- β-iron (Beta ferrite)
- ε-iron (Hexaferrum)
- γ-iron (Gamma ferrite)
- Austenite (γ-iron + carbon in solid solution)
- Cementite (iron carbide, Fe3C)
- Graphite (allotrope of carbon)
- Martensite (metastable microstructure ingredient)
- ε-carbon (transitional carbide, Fe24C)
Important steel microstructure classes
These microstructure ingredients or states are no phases, i.e. they are not in thermodynamic equilibrium and do not fulfill the Gibbs phase rules, yet, they are essential for most of the excellent mechanical properties of steels.
- Spheroidite
- Pearlite (88% ferrite, 12% cementite)
- Bainite
- Ledeburite (austenite-cementite eutectic, 4.3% carbon)
- Tempered martensite (martensite + ferrite + ε-carbon or cementite, or both)
Atom probe tomography (APT) has contributed to our understanding and further design of steels through the detailed analysis of solute behavior, cluster formation, precipitate evolution, and interfacia
Steel studied by Atomprobe-Tomography-Mi[...]
PDF-Dokument [1.4 MB]
Raabe et al. : JOM, Vol. 66, No. 9, 2014,
Here we present recent developments in the field of austenitic steels with up to 18% reduced mass density. The alloys are based on the Fe-Mn-Al-C system.
JOM 66, No. 9, 2014 page 1845 Low-Densit[...]
PDF-Dokument [2.4 MB]
Science-2013-Duarte-372-6.pdf
PDF-Dokument [1.7 MB]
Developing strong, damage-tolerant, and functional steels shapes the backbone for multiple industrial innovations in the fields of energy, transportation, health, safety, machinery, and industrial
infrastructures. Examples are advanced Fe-Cr steels for high-temperature applications in emission-reduced turbines; weight reduced and ultra high strength Fe-Mn steels for light-weight and yet
passenger-protecting mobility; soft magnetic Fe-Si steels, semi-amorphous steels, and iron-based metallic glasses for low-loss electrical motors and wind energy generators; Fe-Cr steels for nuclear
and fusion power plants; or Fe-Cr-Ni stainless steel tubes for direct solar thermal power plants.
The fact that new steels are often 'hidden' behind the final product sometimes conceals the truth that without permanent innovations in this field many industrial products would be impossible.
Awareness of the profound recent advances in steel research sometimes suffers a bit from its engineering success: Although steels are key components in many zero-damage-tolerance safety design parts
(planes, trains, cars, bridges, wind mills, power plants, etc.) they very rarely fail, hence, awareness of the high relevance of steels is not as ubiquitous as the products that are made of it. This
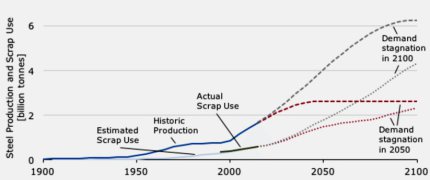
is certain contradiction when considering that the annual steel production exceeds 1.4 Billion tons and that most high-performance steels are complex nanostructured materials.
The applications mentioned above specifically require the development of improved high strength and yet ductile and damage tolerant steels. Most traditional hardening mechanisms, however, such as
solute solution, dislocation multiplication, or precipitates, albeit leading to high strength, often may cause a decrease in ductility, i.e., rendering the material brittle and susceptible for
failure. This phenomenon is sometimes referred to as the inverse strength-ductility problem.
In this context the average grain size and the grain boundaries play an essential role for understanding, designing and manufacturing nanostructured steels with higher ductility.
In many project in my group we specifically address the latter aspect, namely, grain boundaries as defects in advanced high strength steels and their susceptibility to undergo chemical decoration
with solute atoms.
This topic is of high relevance in the context of steel design with respect to damage tolerance because segregation at grain boundaries can profoundly affect their structure and their mechanical
properties: The overall resistance of steels against damage and failure depends on two main material properties. Firstly, steels require during plastic straining a high, if possible, permanent
capability to undergo deformation stimulated strain hardening. This property of the material, such as for instance realized in TRIP (transformation induced plasticity), TWIP (twinning induced
plasticity), and TRIPLEX (weight-reduced austenitic-ferritic with kappa carbides) steels, ensures that any tendency towards macroscopic and microstructural localization associated with geometrical
softening during deformation is compensated by strain hardening that makes the material locally stronger and hence resistant to the further progress of plastic localization zones that might otherwise
become fatal to the structure. Secondly, steels require mechanisms that suppress the formation and specifically the growth of cracks. While tiny flaws, i.e. initial crack nuclei, are
always present in a bulk material, specific attention and understanding has to be placed on mechanisms that help avoiding, branching, deflecting, or even closing cracks that that are capable of
growing or already started to grow.
In the latter context it has been often observed that in high strength steels it are particularly the internal interfaces that may be relatively weak against crack growth. This is due to the
fact that elementary crack growth proceeds by debonding and dislocation emission ate the crack tip, i.e. by decohesion of the atomic bonds and the ability of the material to create a plastic
relaxation zone around the proceeding crack tip. Due to the reduced atomic stacking and hence smaller lattice coherence in the grain boundary zones compared to the bulk grain interiors, cracks
can in high strength steels often proceed more easily along grain boundaries than through the grain interior. This applies particularly to plastically strained steels, often associated with
strain path changes, when the intrinsic strain hardening capacity of the material is gradually exhausted.
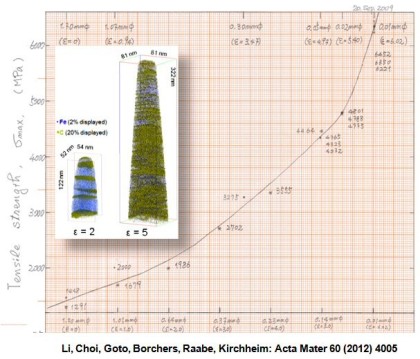
Acta Materialia 60 (2012) 4005
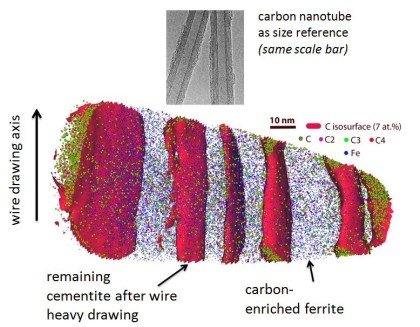
Hypereutectoid steel wires with 6.35 GPa tensile strength after a cold-drawing true strain of 6.02 were annealed between 300 and 723 K. The ultrahigh strength remained upon annealing for 30 min up to a temperature of 423 K but dramatically decreased with further increasing temperature. The reduction of tensile strength mainly occurred within the first 2–3 min of annealing. Atom probe tomography and transmission electron microscopy reveal that the lamellar structure remains up to 523 K. After annealing at 673 K for 30 min, coarse hexagonal ferrite (sub)grains with spheroidized cementite, preferentially located at triple junctions, were observed in transverse cross-sections. C and Si segregated at the (sub)grain boundaries, while Mn and Cr enriched at the ferrite/cementite phase boundaries due to their low mobility in cementite. No evidence of recrystallization was found even after annealing at 723 K for 30 min. The stability of the tensile strength for low-temperature annealing (<473 K) and its dramatic drop upon high-temperature annealing (>473 K) are discussed based on the nanostructural observations.
Li and Raabe Phys Rev Lett vol 113 page [...]
PDF-Dokument [1.6 MB]
PRL 113, 106104 (2014) PHYSICAL REVIEW LETTERS
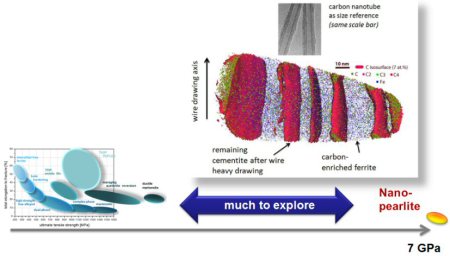
Grain refinement through severe plastic deformation enables synthesis of ultrahigh-strength nano-structured materials. Two challenges exist in that context: First, deformation-driven grain refinement is limited by dynamic dislocation recovery and crystal coarsening due to capillary driving forces; second, grain boundary sliding and hence softening occur when the grain size approaches several nanometers. Here, both challenges have been overcome by severe drawing of a pearlitic steel wire (pearlite: lamellar structure of alternating iron and iron carbide layers). First, at large strains the carbide phase dissolves via mechanical alloying, rendering the initially two-phase pearlite structure into a carbon-supersaturated iron phase. This carbon-rich iron phase evolves into a columnar nanoscaled subgrain structure which topologically prevents grain boundary sliding. Second, Gibbs segregation of the supersaturated carbon to the iron subgrain boundaries reduces their interface energy, hence reducing the driving force for dynamic recovery and crystal coarsening. Thus, a stable cross-sectional subgrain size < 10 nm is achieved. These two effects lead to a stable columnar nanosized grain structure that impedes dislocation motion and enables an extreme tensile strength of 7 GPa, making this alloy the strongest ductile bulk material known.
Mater Sc Engin A 441 (2006) 1 UFG overvi[...]
PDF-Dokument [1.7 MB]
Primer in Composition and Metallurgy of Carbon Steel
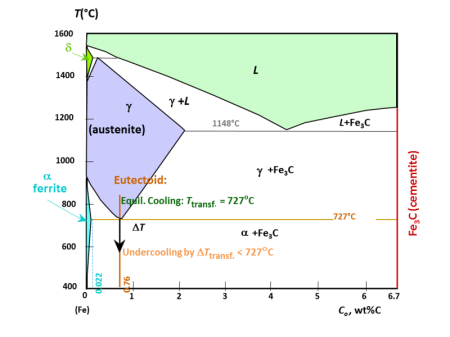
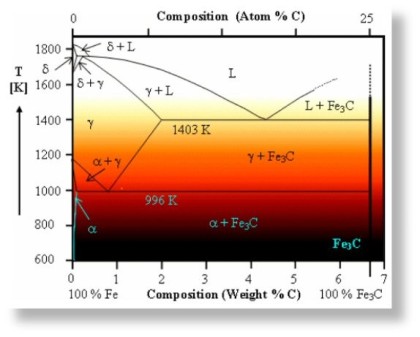
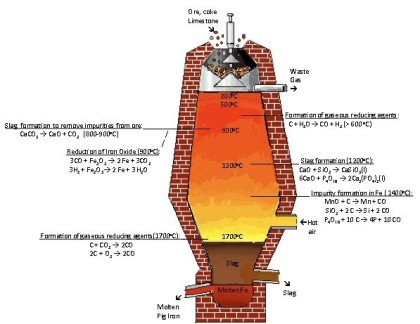
Composition and metallurgy of carbon steels can be best introduced via using the ‘Iron Carbon Diagram’. The diagram shown below is based on the transformation that occurs as a result of slow heating. Slow cooling will reduce the transformation temperatures; for example fast heating and cooling rates ocurring in welding have a significant influence on these temperatures, making the accurate prediction of weld metallurgy using this equilibrium diagram challenging.
Several ingredients, components, phases and atom states with characteristic composition and effect occur in plain carbon steels:
Austenite
This phase is only possible in carbon steel at high temperature. It has a Face Centre Cubic (F.C.C) atomic structure which can contain up to 2% carbon in solution.
Ferrite
This phase has a Body Centre Cubic structure (B.C.C) which can hold very little carbon; typically 0.0001% at room temperature. It can exist as either: alpha or delta ferrite.
Carbon
A very small interstitial atom that tends to fit into clusters of iron atoms. It strengthens steel and gives it the ability to harden by heat treatment. It also may cause problems in
welding, particularly if it exceeds 0.25% as it creates a hard microstructure that is susceptible to hydrogen cracking. Carbon forms compounds with other elements called carbides. Iron
Carbide, Chrome Carbide etc.
Cementite
Unlike ferrite and austenite, cementite is a very hard intermetallic compound consisting of 6.7 wt.% carbon (25 atom %) and the remainder iron, its chemical symbol is Fe3C. Cementite is very
hard, but when mixed with soft ferrite layers its average hardness is reduced considerably. Slow cooling gives course perlite; soft easy to machine but poor toughness. Faster cooling gives very
fine layers of ferrite and cementite; harder and tougher
Pearlite
This is a phase mixture of alternate strips of ferrite and cementite in a single grain. The distance between the plates and their thickness is dependant on the cooling rate of the material; fast cooling creates thin plates that are close together and slow cooling creates a much coarser structure possessing less toughness. The name for this structure is derived from its mother of pearl appearance under a microscope. A fully pearlitic structure occurs at 0.8% Carbon. Further increases in carbon will create cementite at the grain boundaries, which will start to weaken the steel.
Martensite
If Fe-C steel is cooled rapidly from austenite, the F.C.C structure rapidly changes to B.C.C (more specifically to a similar, tetragonally distorted structure) leaving insufficient time for the carbon to form pearlite. This results in a highly distorted structure that has the appearance of fine needles. There is no partial transformation associated with martensite, it either forms or it doesn’t. However, only the parts of a section that cool fast enough will form martensite; in a thick section it will only form to a certain depth, and if the shape is complex it may only form in small pockets. The hardness of martensite is solely dependant on carbon content, it is normally very high, unless the carbon content is exceptionally low.
Allotriomorphic ferrite and allotriomorphic austenite
An allotriomorph is a phase that does not develop its shape according to its otherwise typical external crystallographic form because of a confined transformation condition between earlier formed
crystals, typical of a surrounding matrix alloy or on interfaces.
Typcial exmaples are allotriomorphic ferrite that forms under constrained conditions on austenite grain boundaries when cooling steel. Likewise austenite reversion in a martensitic or ferritic matrix
can lead to allotriomorphic austenite formation.
2011_Acta_Materialia_APT_Steel-Fe-Mn.pdf
PDF-Dokument [2.3 MB]
Development of microstructure and texture of medium carbon steel during heavy warm deformation
Acta Materialia 52 (2004) 2209 texture-s[...]
PDF-Dokument [1.5 MB]
Strength of Steels
Pure iron is a soft material with a yield strength not exceeding some MPa.
Yet, alloys of iron and carbon which are referred to as steels cover a huge strength spectrum from low yield stress levels (around 180 MPa) to very high levels (beyond 6 GPa). These characteristsics make it the most versatile and strong engineering material on earth since 3000 years.
These enormous mechanical properties are usually achieved by the combined use of several strengthening mechanisms, and in such circumstances it is often difficult to quantify the different contributions to the strength.
Like other metals, iron can be strengthened by several basic mechanisms, the most important of which are:
- Work hardening (increase in dislocation density, sometimes also in twin density)
- Solid solution strengthening by interstitial atoms
- Solid solution strengthening by substitutional atoms
- Refinement of grain size and intruduction of twins as additional interfaces
- Dispersion strengthening, including lamellar and random dispersed structures.
The most distinctive aspect of strengthening of iron is the role of the interstitial solutes carbon and nitrogen. These elements also play a vital part in interacting with dislocations, and in combining preferentially with some of the metallic alloying elements used in steels.
Work hardening mechanisms in steel
Work hardening is an important strengthening process in steel, particularly in obtaining high strength levels in rod and wire, both in plain carbon and alloy steels. For example, the tensile strength of a 0.05% C steel subjected to 95% reduction in area by wire drawing, is raised by no less than 550 MPa while higher carbon steels are strengthened by up to twice this amount. Indeed, without the addition of special alloying elements, plain carbon steels can be raised to strength levels above 1500 MPa simply by the phenomenon of work hardening.
Basic work on the deformation of iron has largely concentrated on the other end of the strength spectrum, namely pure single crystals and polycrystals subjected to small controlled deformations. The diversity of slip planes leads to rather irregular wavy slip bands in deformed crystals, as the dislocations can readily move from one type of plane to another by cross slip, provided they share a common slip direction.
The yield stress of iron single crystals are very sensitive to both temperature and strain rate and a similar dependence has been found for less pure polycrystalline iron. Therefore, the temperature sensitivity cannot be attributed to interstitial impurities. It is explained by the effect of temperature on the stress needed to move free dislocations in the crystal, the Peierls-Nabarro stress.
Solid solution hardening in steels using interstitial atoms
The formation of interstitial atmospheres at dislocations requires diffusion of the solute. As both carbon and nitrogen diffuse much more rapidly in iron than substitutional solutes, it is not surprising that strain ageing can take place readily in the range from 20°C to 150°C. Consequently the atmosphere condenses to form rows of interstitial atoms along the cores of the dislocations. These arise because the temperature is high enough to allow interstitial atoms to diffuse during deformation, and to form atmospheres around dislocations generated throughout the stress-strain curve. Steels tested under these conditions also show low ductility`s, due partly to the high dislocation density and partly to the nucleation of carbide particles on the dislocations where the carbon concentration is high. The phenomenon is often referred to as blue brittleness, blue being the interference color of the steel surface when oxidized in this temperature range.
The break away of dislocations from their carbon atmospheres as a cause of the sharp yield point became a controversial aspect of the theory because it was found that the provision of free dislocations, for example, by scratching the surface of a specimen, did not eliminate the sharp yield point. An alternative theory was developed which assumed that, once condensed carbon atmospheres are formed in iron, the dislocations remain locked, and the yield phenomena arise from the generation and movement of newly formed dislocations.
To summarize, the occurrence of a sharp yield point depends on the occurrence of a sudden increase in the number of mobile dislocations. However, the precise mechanism by which this takes place will depend on the effectiveness of the locking of the pre-existing dislocations. If the pinning is weak, then the yield point can arise as a result of unpinning. However, if the dislocations are strongly locked, either by interstitial atmospheres or precipitates, the yield point will result from the rapid generation of new dislocations.
Under conditions of dynamic strain ageing, where atmospheres of carbon atoms form continuously on newly-generated dislocations, it would be expected that a higher density of dislocations would be needed to complete the deformation, if it is assumed that most dislocations which attract carbon atmospheres are permanently locked in position.
Austenite can take into solid solution up to 10% carbon, which can be retained in solid solution by rapid quenching. However, in these circumstances the phase transformation takes place, not to ferrite but to a tetragonal structure referred to as martensite. This phase forms as a result of diffusion less shear transformation leading to characteristic laths or plates.
If the quench is sufficiently rapid, the martensite is essentially a supersaturated solid solution of carbon in a tetragonal iron matrix, and as the carbon concentration can be greatly in excess of the equilibrium concentration in ferrite, the strength is raised very substantially. High carbon martensites are normally very hard but brittle, the yield strength reaching as much as 1500 MPa; much of this increase can be directly attributed to increased interstitial solid solution hardening, but there is also a contribution from the high dislocation density, which is characteristic of martensitic transformations in iron-carbon alloys.
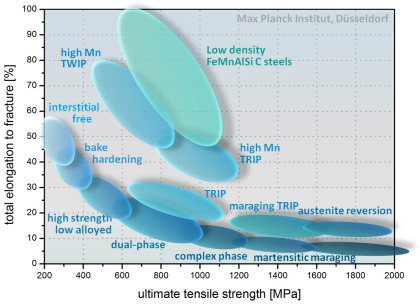
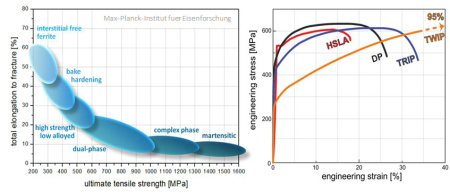
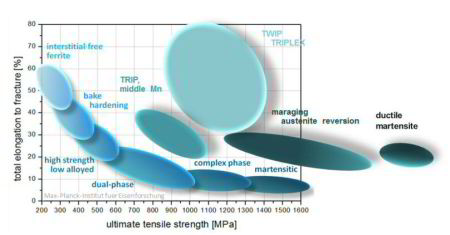
Inverse strength-ductility relationship for different types of steels. The introduction of special deformation mechanisms which active additional strain hardening capacity at high strains such as the TRIP or TWIP effects can lead to novel metallic materia
Rapid prototyping of novel TRIPLEX steels: Compositional and thermo-mechanical high throughput bulk combinatorial design of structural materials based on the example of 30Mn–1.2C–xAl triplex steel
Acta-Mater-2012-RAP--30Mn-triplex-steels[...]
PDF-Dokument [1.3 MB]
Further reprints on steels :