Hydrogen and hydrogen embrittlement in metallic alloys
What is Hydrogen Embrittlement ?
Phenomenologically hydrogen embrittlement is the result of the absorption of hydrogen by susceptible metals, leading to the delayed or sudden loss of ductility and reduction of load-bearing capability. Stress loading even far below the macroscopic yield püoint of the embrittled material can result in cracking and sudden or delayed catastrophic brittle failure. Hydrogen embrittlement is also referred to as hydrogen-induced cracking or hydrogen attack. It belongs to the class of physics-based corrosion phenomena. It is one the most complex materials phenomena because hydrogen is very hard to detect, hence, structure-property relations are often established on a phenomenological basis only.
How does Hydrogen Embrittlement work ?
Hydrogen embrittlement of steels and related transition metall alloys is attributed to a variety of possible mechanisms. At room temperature, hydrogen atoms can be absorbed into the metal lattice and diffuse through the grains and other lattice defects. The absorbed hydrogen may be present either as an atomic or in recombined molecular form. Regardless of the form, the atoms or molecules combine to form small bubbles at metal grain boundaries. These bubbles can act as pressure-concentrators, building up pressure between the metal grains. The pressure can increase to levels where the metal has reduced ductility, causing the formation of minute cracks inside the material. The cracking is intergranular. That is, the crack grows along the metal grain boundaries.
Another mechanism that is often discussed in that context is that atomic hydrogen can directly segregate to dislocations and grain boundaries, reducing the cohesion of the metallic lattice at these positions. An essential aspect is also that hydrogen can decorate vacancies, substantially reducing their formation energy, hence, the vacancy density can be drastically enhanced upon hydrogen loading. These vacancies can promote the formation of nano - voids, void clusters at interfaces, or bigger voids that lead to crack initiation. Another mechanism that is often discussed in the field of hydrogen embrittlement is that hydrogen atoms decorate dislocations and promote stress shielding effects among them. This leads to locally higher dislocation densities for instance in pileup configurations. A further mechanism that seems to be of relevance is that the decoration of hydrogen atoms to dislocations seems to promote also their mobility.
What is the Delayed Facture Phenomenon in Hydrogen Embrittlement?
Understanding hydrogen-assisted embrittlement of advanced structural materials is essential for enabling future hydrogen-based energy industries. A crucially important phenomenon in this context is the delayed fracture in high-strength structural materials. Factors affecting the hydrogen embrittlement are the hydrogen content, residual stress (as well as applied stress) and microstructure. These factors can all lead to a critical condition for hydrogen embrittlement through specific mechanisms, e.g. hydrogen-induced decohesion, hydrogen-enhanced strain-induced vacancy formation and hydrogen-enhanced localized plasticity. In our group at the Max-Planck Institut in Düsseldorf we study the mechanisms of hydrogen embrittlement in a new class of steels, namely Fe–Mn–C twinning-induced plasticity (TWIP) steels. These materials present a new group of advanced high-strength and formable austenitic steels with high potential for automotive forming applications. Moreover, energy-related infrastructures and pipelines are envisaged as future applications of these steels.
Which are the most important Damage Mechanisms in Hydrogen Embrittlement?
The hydrogen - promoted embrittlement of steels depends on the kind of steel and also on the hydrogen charging conditions. Phases such as soft ferrite, hard C-martensite, maraging-martensite,
austenite etc. might follow different mechanism combinations, kinetics, and trapping spectra.
Several important mechanisms were proposed:
Hydride-induced embrittlement, i.e. effect of a second phase
Hydrogen-enhanced decohesion, HEDE, leading to brittle fracture
Hydrogen-enhanced localized plasticity, HELP, leading to ductile fracture
Hydrogen-enhanced vacancy formation and stabilization through the defectant mechanism
My current understanding of failure in many pipeline and sheet steels relates to the interaction among these different phenomena, characterized by these steps:
i) H enhances dislocation formation leading to high dislocation densities and dense configurations;
ii) the dislocation interactions can create vacancies (e.g. jog drag and the associated climb osmotic effects);
iii) these vacancies get decorated and stabilized by hydrogen (it reduces their free energy of formation due to the defectant theory);
iv) These nanovoids can coalesce and condensate at interfaces, interface junctions, microbands, twins, or cell walls;
v) The voids can grow further to form larger voids.
Which are the key factors and boundary conditions that can lead to hydrogen embrittlement of engineering components?
There are three main required aspects that promote failure due to hydrogen embrittlement:
A susceptible material, typcially a high strength metallic alloy
Exposure to an environment that contains hydrogen and promotes formation of atomic hydrogen. This can be permanent (sour gas environment, hydrogen proplled industry cycles) or
non-permanent (galvanic coating)
The presence of internal tensile stresses due to residual and/or applied stresses.
How does hydrogen enter the material
Hydrogen can enter and diffuse through a metal alloy surface at ambient or elevated temperatures. This can occur during various manufacturing and assembly operations or when the component is in service, i.e. anywhere that the metal comes into contact with atomic or molecular hydrogen.
Typical industry processes that can lead to hydrogen embrittlement include: galvanic zinc coating, phosphating, acid pickling, electroplating and arc welding. During these processes, there is a possibility of absorption of hydrogen by the material. For example, during arc welding, hydrogen is released from moisture (for example in the coating of the welding electrodes; to minimize this, special low-hydrogen electrodes are used for welding high-strength steels).
During use, hydrogen can be introduced into metal as a result of corrosion, chemical reactions of metal with acids, or with other chemicals—notably hydrogen sulfide in sulfide stress cracking.
Hydrogen embrittlement of TWIP steels and general austenitic steels
Hydrogen embrittlement of austenitic steels is of high interest because of the potential use of these materials in hydrogen-energy related infrastructures. In order to elucidate the associated hydrogen embrittlement mechanisms, the mapping of heterogeneities in strain, damage (crack/void), and hydrogen and their relation to the underlying microstructures is a key assignment in this field.
Specifically mapping the connection between microstructure heterogeneity
and the associated hydrogen trapping at similar spatial resolution opens a novel pathway to identify hydrogen embrittlement mechanisms in complex alloys.
One of the materials classes that is expected to be applied for energy-related structure parts are austenitic steels with high Mn content. In particular, twinning-induced plasticity (TWIP)
high Mn austenitic steels are well known for an exceptional balance of ductility and strength with less hydrogen susceptibility compared to ferritic steels with a similar
strength.
The hydrogen embrittlement phenomenon has been observed under severe mechanical deformation and hydrogen charging conditions such as delayed fracture testing in a deep drawn cup
or tensile testing during hydrogen charging at a high current density. Fracture was in such cases caused by various metallurgical factors e.g. by the formation and failure of deformation twins.
The importance of deformation twins on hydrogen embrittlement of austenitic steels has been recently studied. It was found that they can act as crack initiation sites and enable crack
propagation.
Microstructure-sensitive hydrogen mapping: locally resolved observation of hydrogen and its damaging effects
Microstructure-sensitive mapping of hydrogen in TWIP steels and generalla in related metallic alloys has been conducted by using microprinting experiments, which enable visualization of
hydrogen emission from a sample through reduction of silver ions in gelatin-based AgBr emulsion. The microprinting technique demonstrated that hydrogen is indeed localized at deformation twins,
hence, promoting the initiation of hydrogen embrittlement. Also, from our previous experiments we suggested that the hydrogen localization at/near deformation twins requires local plastic straining
at/before deformation twins. These facts indicate that the hydrogen-assisted twin boundary cracking of TWIP steels is a complex phenomenon including both, local plastic straining and local
hydrogenation. To better understand the influence of deformation twins on the hydrogen embrittlement phenomenon, it is thus essential to map the spatial hydrogen distribution through a high
resolution detection approach that is sufficiently microstructure and concentration sensitive. It should be noted here that the microprinting technique is only sensitive to hydrogen at relatively
high concentration levels, or more precisely at relatively high emission rates. It has, therefore, to be applied directly after a sample is charged with hydrogen. It will be shown here that hydrogen
is critical for prolonged times after charging. Hence, the findings obtained by microprinting needed to be confirmed also for longer times after charging by a more sensitive technique. In the last
years several promising novel approaches have been reported for the localized resolved and sensitive detection of hydrogen.
One is a direct electrochemical detection via a capillary cell, developed by Suter et al. which, however, does not provide the resolution required here. Another approach is the use of Kelvin probe
techniques, which allow detection of hydrogen in a material by means of the change ofwork function caused by the hydrogen entering the oxide at the surface. Studies at quite high resolution have been
carried out with Scanning Kelvin Probe ForceMicroscopy (SKPFM), where diffusion profiles of hydrogen have been mapped successfully at relatively high
resolution at cross section of samples after hydrogen charging.
However, a direct quantification is not possible by this method, due to the complex dependence of the work function of oxide on different defect states in the oxides. For the same reason this
approach is not suitable to provide reliable information on hydrogen at different features of the microstructure, because different oxides show a different dependence on hydrogen. A new approach by
applying a thin palladium layer eliminates this problem. More specifically, the SKPFM with a thin palladium layer is a novel and efficient method to very sensitively analyze local hydrogen
concentrations down to levels well below 0.01 atom ppm at spatial resolutions as small as several tens of nanometers. The SKPFM can hence be used for detecting the hydrogen distribution with high
sensitivity, since the potential measured by the SKPFM on the palladium that has been deposited as a thin layer on the hydrogen charged sample surface correlates logarithmically with the hydrogen
content in the palladium. Because of this Nernstian-like behavior, which is similar to a hydrogen electrode, as also reported for immersed palladium metal, we also refer to the work function as
electrode potential. In fact, as discussed previously, the surface of the palladium is, even in dry nitrogen atmosphere, still covered by an ultrathin water layer, which together with the hydrogen in
the palladium leads to the formation of an hydrogen electrode “in the dry”. By exploiting this effect, SKPFM was successfully applied to the spatially resolved detection of hydrogen in
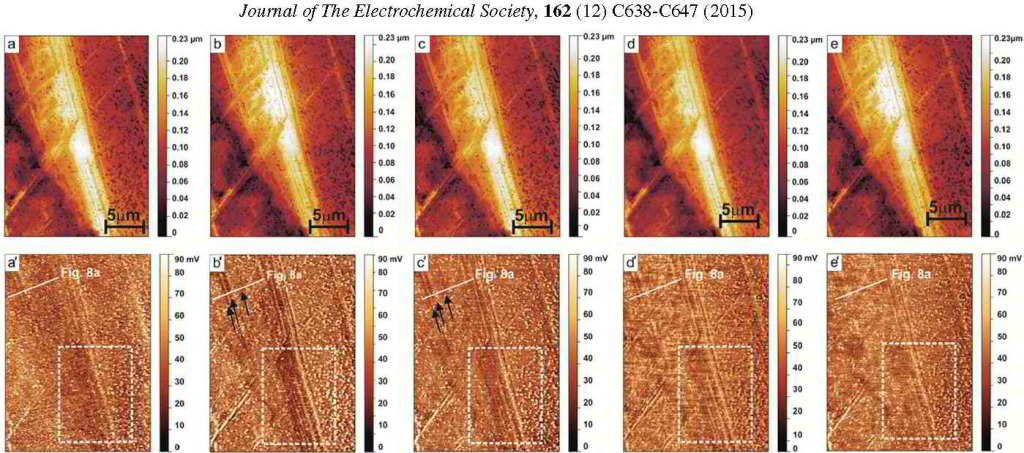
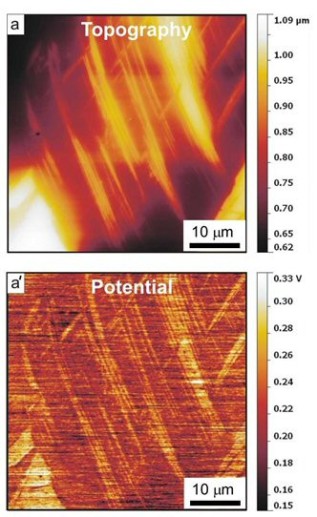
ferrite/austenite duplex stainless steels. More specific in this project the hydrogen distribution in a hydrogen-charged Fe-18Mn-1.2C (wt%) twinning-induced plasticity austenitic steel was studied by
Scanning Kelvin Probe Force Microscopy (SKPFM). We observed that 1–2 days after the hydrogen-charging, hydrogen showed a higher activity at twin boundaries than inside the matrix. This result
indicates that hydrogen at the twin boundaries is diffusible at room temperature, although the twin boundaries act as deeper trap sites compared to typical diffusible hydrogen trap sites such as
dislocations. After about 2 weeks the hydrogen activity in the twin boundaries dropped and was indistinguishable from that in the matrix. These SKPFM results were supported by thermal desorption
spectrometry and scanning electron microscopic observations
of deformation-induced surface cracking parallel to deformation twin boundaries. With this joint approach, two main challenges in the field of hydrogen embrittlement research can be overcome, namely,
the detection of hydrogen with high local and chemical sensitivity and the microstructure-dependent and spatially resolved observation of the kinetics of hydrogen desorption.
Further reading on spatially and time resolved observation of Hydrogen in a Twinning-Induced Plasticity Steel by Use of Scanning Kelvin Probe Force Microscopy
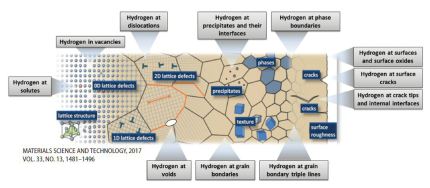
Recent progress in microstructural hydrogen mapping in steels: quantification, kinetic analysis, and multi-scale characterisation
Microstructure-specific hydrogen mapping has been recognised as the most important challenge on the pathway towards a better understandingof thenature of hydrogen embrittlement in
metallic alloys, specifically in steels. Local presence of hydrogen is challenging to map due to its low mass, huge embrittling effect already at low concentrations, and high
diffusivity in presence of compositional, thermal and mechanical gradients. For instance, the average hydrogen content in steels is of the order of 10−1 to 100 mass ppm, and some
of
the hydrogen is diffusible even at room temperature – an effect further enhanced in the presence of stress localisation effects. These complications render conventional chemically and
spatially sensitive mapping methods such as energy dispersive X-ray spectroscopy or wavelength dispersive X-ray spectroscopy not readily applicable in the context of hydrogen
embrittlement.
To enable both, spatially resolving and microstructure-sensitive hydrogen mapping, various approaches have been used, e.g. autoradiography, scanning photoelectrochemical microscopy,
scanning electrochemical microscopy, secondary ion mass spectroscopy (SIMS), silver reduction and decoration, the hydrogen microprint technique (HMT), scanning Kelvin probe
force
microscopy (SKPFM), atom probe tomography (APT), prompt activation analysis, proton–proton (pp) scattering , and neutron radiography. Additionally, a quantitative method for
determining the hydrogen content at specific microstructural constituents with a characteristic trapping depth such as dislocations is available in terms of thermal desorption spectrometry
(TDS). Although this method does not provide spatially resolving mapping, hydrogen desorption rate profiles detected by TDS are microstructure-sensitive.
In the paper placed below we give an overview of recent progress in microstructure-specific hydrogen mapping techniques. The challenging nature of mapping hydrogen with high spatial
resolution, i.e. at the scale of finest microstructural features, led to the development of various methodologies: thermal desorption spectrometry, silver decoration, the hydrogen
microprint technique, secondary ion mass spectroscopy, atom probe tomography, neutron radiography, and the scanning Kelvin probe. These techniques have different characteristics regarding
spatial and temporal resolution associated with microstructure-sensitive hydrogen detection. Employing these techniques in a
site-specific manner together with other microstructure probing methods enables multi-scale, quantitative, three-dimensional, high spatial, and kinetic resolution hydrogen mapping,
depending on the specific multi-probe approaches used. Here, we present a brief overview of the specific characteristics of each method and the progress resulting from their combined
application to the field of hydrogen embrittlement.
Mater Sc Techn 2017 Recent progress in m[...]
PDF-Dokument [4.9 MB]
Journal of The Electrochemical Society, 162 (12) C638-C647 (2015)
Spatially and Kinetically Resolved Mapping of Hydrogen in a Twinning-Induced Plasticity Steel by Use of Scanning Kelvin Probe Force Microscopy
Motomichi Koyama, Asif Bashir,Michael Rohwerder,Sergiy V. Merzlikin, Eiji Akiyama, Kaneaki Tsuzaki, and Dierk Raabe
J. Electrochem. Soc. 2015-Koyama vol 162[...]
PDF-Dokument [1.9 MB]
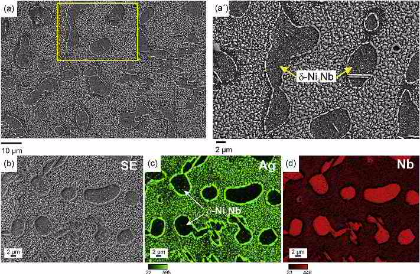
Hydrogen embrittlement of Nickel alloys and superalloys
Here we investigated the hydrogen distribution and desorption behavior in an electrochemically hydrogen-charged binary Ni-Nb model alloy to study the role of d phase in hydrogen embrittlement of
alloy 718. We focus on two aspects, namely, (1) mapping the hydrogen distribution with spatial resolution enabling the observation of the relations between desorption profiles and
desorption sites; and (2) correlating these observations with mechanical testing results to reveal the degradation mechanisms. The trapping states of hydrogen in the alloy were globally
analyzed by Thermal Desorption Spectroscopy (TDS). Additionally, spatially resolved hydrogen mapping was conducted using silver decoration, Scanning Kelvin Probe Force Microscopy (SKPFM) and
Secondary Ion Mass Spectrometry (SIMS): The Ag decoration method revealed rapid effusion of hydrogen at room temperature from the gamma-matrix. The corresponding kinetics was resolved in
both, space and time by the SKPFM measurements. At room temperature the hydrogen release from the FCC gamma-matrix steadily decreased until about 100 h and then was taken over by the d
phase from which the hydrogen was released much slower. For avoiding misinterpretation of hydrogen signals stemming from environmental effects we also charged specimens with deuterium. The
deuterium distribution in the microstructure was studied by SIMS. The combined results reveal that hydrogen dissolves more preferably inside the g-matrix and is diffusible at room
temperature while the delta-phase acts as a deeper trapping site for hydrogen. With this joint and spatially resolving approach we observed the microstructure- and time-dependent
distribution and release rate of hydrogen with high spatial and temporal resolution. Correlating the obtained results with mechanical
testing of the hydrogen-charged samples shows that hydrogen enhanced decohesion (HEDE) occurring at the delta/matrix interfaces promotes the embrittlement.
Acta Materialia 109 (2016) 69-81
Multi-scale and spatially resolved hydrogen mapping in a Ni-Nb model alloy reveals the role of the d phase in hydrogen embrittlement of alloy 718
Acta Materialia 109 (2016) 69 Multiscale[...]
PDF-Dokument [2.5 MB]
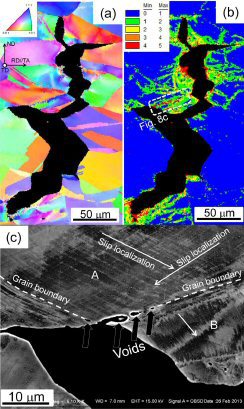
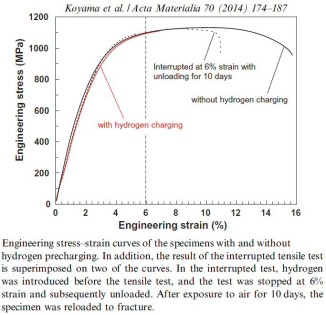
Hydrogen embrittlement of a precipitation-hardened Fe-26Mn-11Al-1.2C (wt.%) weight reduced austenitic steel
Hydrogen embrittlement of a precipitation-hardened Fe-26Mn-11Al-1.2C (wt.%) austenitic steel was examined by tensile testing under hydrogen charging and thermal desorption analysis.
While the high strength of the alloy (>1 GPa) was not affected, hydrogen charging reduced the engineering tensile elongation from 44 to only 5%. Hydrogen-assisted cracking mechanisms
were studied via the joint use of electron backscatter diffraction analysis and orientation-optimized electron channeling contrast imaging. The observed embrittlement was mainly due to two
mechanisms, namely, grain boundary triple junction cracking and
slip-localization-induced intergranular cracking along micro-voids formed on grain boundaries. Grain boundary triple junction cracking occurs preferentially, while the microscopically
ductile slip-localization-induced intergranular cracking assists crack growth during plastic deformation resulting in macroscopic brittle fracture appearance.
Hydrogen embrittlement of a precipitation-hardened Fe-26 Mn-11 Al-1.2 C (wt.%) austenitic steel was examined by tensile testing under hydrogen charging and thermal desorption analysis.
International Journal of Hydrogen Energy
39 ( 2014) 4634-4646
Intern J Hydrogen Energy - Hydrogen embr[...]
PDF-Dokument [1.9 MB]
Can Hydrogen / Deuterium be observed at atomic scale ?
In this project we investigated an electrolytic route for hydrogen charging of metals and its detection in Atom Probe Tomography (APT) experiments. We charge an austenitic Fe-30Mn-8Al-1.2C (wt.%)
weight reduced high-Mn steel and subsequently demonstrate the detectability of deuterium in an APT experiment. The experiment is repeated with a deposited Ag film
upon an APT tip of a high-Mn steel. It is shown that a detectable deuterium signal can be seen in the high-Mn steel, and a D:H ratio of 0.84 can be reached in Ag films. Additionally, it was found
that the predicted time constraint on detectability of D in APT was found to be lower than predicted by bulk diffusion for the high-Mn steel.
interna. J. Hydrogen Energy volume 39 ( 2014 ) page 12221:
Intern Journal Hydrogen Energy v...2014)[...]
PDF-Dokument [541.6 KB]
Can hydrogen enhance the strength and ductility of equiatomic high entropy alloys? Example of the Cantor Alloys
Metals are key materials for modern manufacturing and infrastructures as well as transpot and energy solutions owing to their strength and formability. These properties can severely
deteriorate when they contain hydrogen, leading to unpredictable failure, an effect called hydrogen embrittlement. Here we
report that hydrogen in an equiatomic CoCrFeMnNi high-entropy alloy (HEA) leads not to catastrophic weakening, but instead increases both, its strength and ductility. While HEAs originally aimed
at entropy-driven phase stabilization, hydrogen blending acts opposite as it reduces phase stability.
This effect, quantified by the alloy’s stacking fault energy, enables nanotwinning which increases the material’s work-hardening. These results turn a bane into a boon: hydrogen does not
generally act as a harmful impurity, but can be utilized for tuning beneficial hardening mechanisms. This opens new pathways for the design of strong, ductile, and hydrogen tolerant
materials.
Scientific Reports 7: 9892 | DOI:10.1038/s41598-017-10774-4
Luo_et_al-2017-Scientific_Reports.pdf
PDF-Dokument [2.6 MB]
Corrosion Science 136 (2018) 403-408
Corrosion Science 2018 Hydrogen embrittl[...]
PDF-Dokument [3.3 MB]
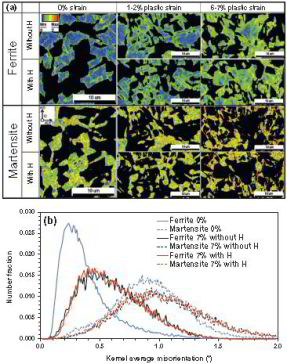
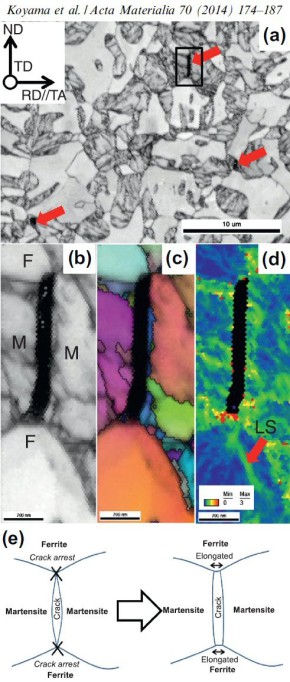
Hydrogen embrittlement in dual-phase (DP) steel
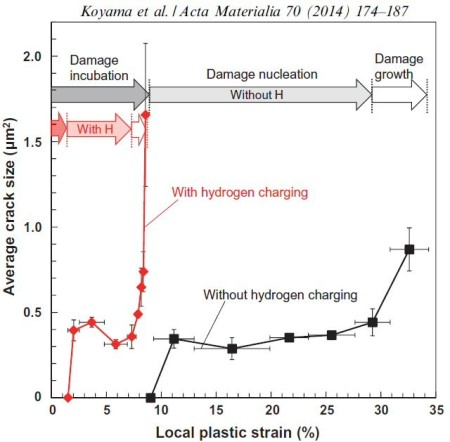
Hydrogen embrittlement affects high-strength ferrite/martensite dual-phase (DP) steels. The associated micromechanisms which lead to failure have not been fully clarified yet. Here we present
a quantitative micromechanical analysis of the microstructural damage phenomena in a model DP steel in the presence of hydrogen. A high-resolution scanning electron microscopy-based damage
quantification technique has been employed to identify strain regimes where damage nucleation and damage growth take place, both with and without hydrogen precharging. The mechanisms
corresponding to these regimes have been investigated by employing post-mortem electron channeling contrast imaging and electron backscatter diffraction analyses, as well as additional in situ
deformation experiments. The results reveal that damage nucleation mechanism (i.e. martensite decohesion) and the damage growth mechanisms (e.g. interface decohesion) are both promoted by
hydrogen, while the crack-arresting capability of the ferrite is significantly reduced. The observations
are discussed on the basis of the hydrogen-enhanced decohesion and hydrogen-enhanced localized plasticity mechanisms. We discuss
corresponding microstructure design strategies for better hydrogen-related damage tolerance of DP steels.
Acta Materialia 70 (2014) 174-187
Hydrogen embrittlement affects high-strength ferrite/martensite dual-phase (DP) steels. The associated micromechanisms which lead to failure have not been clarified. Here we study the microstructural
Acta Materialia 70 (2014) 174 Hydrogen d[...]
PDF-Dokument [4.3 MB]
General overview of dual-phase (DP) steels inclusing hydrogen embrittlement
Dual-phase (DP) steel is the flagship of advanced high-strength steels, which
were the first among various candidate alloy systems to find application in
weight-reduced automotive components. On the one hand, this is a metal-
lurgical success story:Lean alloying and simple thermomechanical treatment
enable use of less material to accomplish more performance while comply-
ing with demanding environmental and economic constraints. On the other
hand, the enormous literature onDP steels demonstrates the immense com-
plexity ofmicrostructure physics inmultiphase alloys: Roughly 50 years after
the first reports on ferrite-martensite steels, there are still various open sci-
entific questions. Fortunately, the last decades witnessed enormous advances
in the development of enabling experimental and simulation techniques, sig-
nificantly improving the understanding of DP steels. This review provides
a detailed account of these improvements, focusing specifically on (a) mi-
crostructure evolution during processing, (b) experimental characterization
of micromechanical behavior, and (c) the simulation of mechanical behavior,
to highlight the critical unresolved issues and to guide future research efforts.
Annu. Rev. Mater. Res. 2015. 45:391–431
C.C. Tasan, M. Diehl, D. Yan, M. Bechtold, F. Roters, L. Schemmann, C. Zheng, N. Peranio, D. Ponge, M. Koyama, K. Tsuzaki, and D. Raabe
Annual Reviews Materials Research 2015. [...]
PDF-Dokument [4.1 MB]
Hydrogen-assisted failure in a twinning-induced plasticity steels
Understanding hydrogen-assisted embrittlement of advanced steels is essential for enabling hydrogen-based energy industries. An important phenomenon in this context is the delayed fracture in
high-strength structural materials. Factors affecting the hydrogen embrittlement have been reported to be the hydrogen content, residual stresses as well as applied stresses and the underlying
microstructures. These factors can all lead to critical conditions for hydrogen embrittlement through specific mechanisms, e.g. hydrogen-induced decohesion, hydrogen-enhanced strain-induced vacancy
formation and hydrogen-enhanced localized plasticity.
In this project we have studied the mechanisms of hydrogen embrittlement in a new and essential class of high strength steels, namely Fe–Mn–C
twinning-induced plasticity (TWIP) steels. These materials present a new group of advanced high-strength and formable austenitic steels with high potential for automotive and related
forming applications. Moreover, energy-related infrastructures and pipelines are envisaged as future applications of these steels. Hydrogen embrittlement of TWIP steels was observed in the form of delayed fracture tests in cup-drawn specimens under air similar as was observed before in certain austenitic stainless steels and
also in tensile tests with cathodically hydrogen-charged specimens. Clarifying the mechanism of the respective hydrogen embrittlement of TWIP steels is an essential pending problem. The effects of
hydrogen content and residual stress as well as of the applied stress have already been examined thoroughly in that context. However, the effect of the corresponding microstructures is still under
debate due to the complexity of the phenomena and boundary conditions involved. Microstructures affecting the hydrogen embrittlement of TWIP steels have been reported to be dislocation density,
martensite content, and deformation twins. In contrast, there have been only few reports on the effect of deformation twins, which are indispensable lattice defects governing the excellent
formability of TWIP steels.
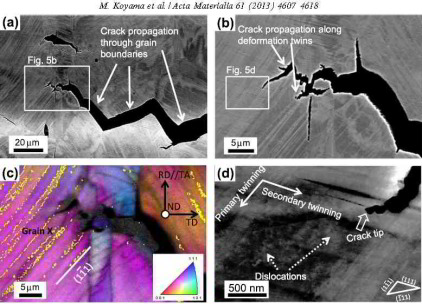
Deformation twinning is a characteristic phenomenon in face-centered cubic (fcc) materials with low stacking fault energy, and is
reported to cause embrittlement even in non-hydrogen-charged steels. In a previous study, the hydrogen-assisted cracking of a Fe–18Mn–1.2C TWIP
steel (wt.%) was found to occur at deformation twin boundaries as well as at grain boundaries, although the precise mechanism was not revealed. Deformation twin boundary cracking was
observed by electron channeling contrast imaging (ECCI), which is an excellent method for detecting fine twins. Since ECCI enables
the visualization of deformation twins at a wide field of view compared to transmission electron microscopy, it is a suitable technique for investigating the correlation between deformation twinning
and cracking on a mesoscopic scale. In particular, crack propagation associated with the occurrence of deformation twinning is expected to be clarified by the use of ECCI. For this purpose, we used a
Fe–18Mn–1.2C steel that is known to show high strength in conjunction with exceptional elongation. This specific TWIP steel does not contain any martensite even after it fractures at ambient
temperature (294 K) due to its high carbon concentration; however, hydrogen embrittlement occurs when it is exposed to tensile testing under hydrogen charging.
The aim of this study thus was to reveal how deformation twinning contributes to hydrogen-assisted cracking along both non-coherent grain boundaries and sigma 3 coherent twin boundaries in a stable
austenitic TWIP steel using ECCI.
The main novelty of this approach lies in two fields: first, we conduct the tensile test directly under permanent electrochemical hydrogen charging; secondly, we use ECCI to map microstructure
changes that result from hydrogen
charging. This combined experimental approach enables tracking the microstructure at a wide field of view under minimum sample preparation requirements for imaging dislocation, twin and interface
structures. This approach has advantages over the use of transmission electron microscopy owing to the wide field of view that ECCI offers.
Acta Materialia 61 (2013) 4607 hydrogen [...]
PDF-Dokument [1.6 MB]
Hydrogen embrittlement was observed in an Fe–18Mn–1.2C (wt.%) steel. The tensile ductility was drastically reduced by hydrogen charging during tensile testing. The fracture mode was mainly intergr
scripta-mater-hydrogen-induced-cracking-[...]
PDF-Dokument [693.6 KB]
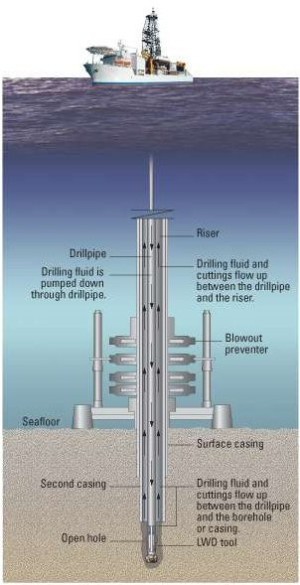
Materials used in deep sea offshore applications
Plain Carbon Steel with strength between 500 and 800 MPa
Austenitic Stainless Steel
Copper Alloys
Nickel/Aluminium Bronze
Duplex and Super Duplex Stainless Steel
Super Austenitic Stainless Steel
Titanium
Nickel alloys
Alloy 400/K500, Alloy 625/825
Glass reinforced epoxy (GRE)
During the next decade the most severe new challenges of oil and gas exploration are in the domain of deep wells, specifically
those in deep seawater. Compared to shallow gas and oil wells, sub deep sea water wells require alloy design and equipment built with
more high performance steels and nickel base alloys. Wells are categorized either being sweet or sour.
Sweet wells being only mildly corrosive, while sour wells are highly corrosive and contain hydrogen sulfide, carbon dioxide, chlorides and free sulphur.
In addition different levels of corrosive conditions are compounded by temperatures up to 260°C and pressures up to 25000 psi (172 MPa)
and generally deep wells generally have higher temperatures and pressures. Alloy selection is hence especially critical for such sour gas dep sea wells.
Also, the alloy and equippment design must under such boundary conditions be absolutely damage tolerant as the deep sea horozon catastrophy has recently shown.
Consequently the next generation materials of primary choice must be highly sour gas corrosion resistant, cost effective, reliable and have the
required strength for the deep sea well conditions. As these conditions become more severe, material selection changes from carbon steels for sweet wells
to duplex (austenitic-ferritic) stainless steel, to nickel based alloys such as Incoloy alloys 825 and 925, Inconel alloys 725HS and 725.