introduction to Titanium Alloys
Gum Titanium Alloys: High Formability of Near-Beta Ti Alloys
Hip Implants and Teeth: Advanced Titanium Alloys for Biomedical Applications
Mechanical Properties of Titanium Alloys
Literature on Titanium Alloys
Titanium is widely distributed throughout the whole universe such as stars and interstellar dust. After Al, Fe and Mg, titanium is the fourth most abundant of structural metals and is the ninth most abundant element on the earth.
Titanium exists in most minerals such as ilmenite (FeTiO3); rutile (TiO2); arizonite (Fe2Ti3O9); perovskite (CaTiO3) and titanite (CaTiSiO5).
Titanium offers a unique property spectrum owing to the combination of high strength, stiffness, toughness, low density, and good corrosion resistance. These properties are enabled by a wide variety of titanium alloys ranging from applications at very low to elevated temperatures. This spectrum enables weight savings in multiple key aerospace applications and other high-performance applications in the medical, chemcial and car industry.
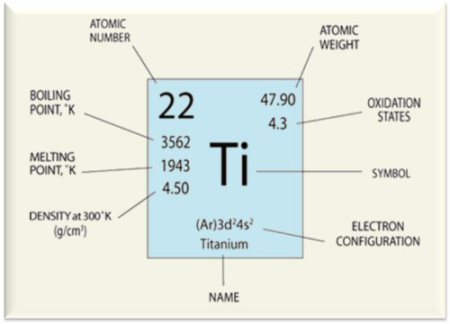
The atomic weight of titanium is 47.88. Titanium is elastically stiff (around 115 GPa Young's modulus), intrinsically strong, lightweight, corrosion resistant and highly abundant in nature. Titanium and its alloys possess tensile strengths ranging between 210-1380 MPa, which approaches the strengths found in many complex steels.
Titanium is a low-density element (approximately 60% of the density of iron) that can be strengthened by solute alloying, second phase hardening, and plastic deformation. Titanium is nonmagnetic and has good heat-transfer properties. Its coefficient of thermal expansion is somewhat lower than that of steels and less than half that of aluminum.
One of its important properties is its high melting point of 1725°C, i.e. nearly 200°C above that of steel and more than 1000°C above that of aluminum.
Titanium can be passivated, and thereby its alloys exhibit a high degree of immunity to attack by most mineral acids and chlorides. Titanium is nontoxic and generally biologically compatible with human tissues, minerals and bones. The excellent corrosion resistance and biocompatibility coupled with excellent strength make titanium and its alloys useful in chemical and petrochemical applications, marine environments, and biomaterial applications.
Titanium is not a good conductor of electricity. If the conductivity of copper is taken as 100%, titanium has 3.1%.
Electrical resistance is the opposition a material presents to the flow of electrons. Since titanium is a poor conductor, it follows that it is a fairly good resistor.
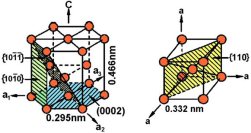
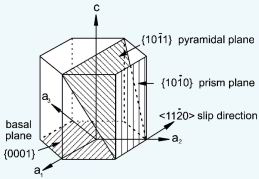
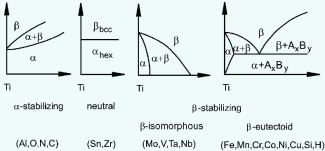
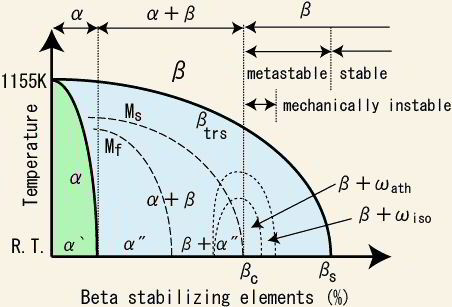
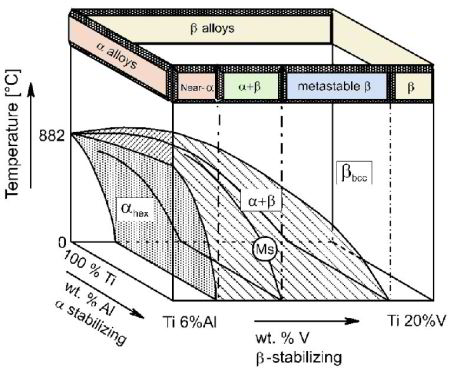
For pure Ti the low temperature phase (α -Ti) is a near hexagonal closed packed (HCP) crystal structure which undergoes an allotropic transformation at 882°C to the high temperature phase ( β-Ti) with body centered cubic (BCC) crystal structure. Ti-alloys are hence classified into three types, namely, α-alloys, β -alloys and α - β -alloys. These alloys are stabilized by solute elements that have strong effect on the transformation temperature. Elements increasing the transformation temperature are referred to as α -stabilizers, while elements that decrease it, thereby increasing the β- phase region, are referred as the β -stabilizers. The most common α -stabilizers are Al, O, C and N, whereas many heavy and high melting transition elements such as particularly Fe, Cr, Nb, Mo, Ta, V, and W are β -stabilizers. For β -stabilizers, a minimum concentration β is required to fully stabilize the β -phase following a quench from the high temperature.
Most titanium alloys used in structural applications in the aerospace, energy and chemical industries consist of two-phase mixtures of α and β phases combined in different morphologies and relative volume fractions. Two-phase α + β alloys offer a wide range of combinations of strength, toughness and high temperature properties up to ~600°C. Solid state phase transformations are the main factors determining the functional characteristics of these alloys. Manipulation of the mechanical properties depends on the effect of alloying additions and thermo-mechanical processing on the stability and physical and mechanical behavior of the two phases, both individually and in a variety of microstructural combinations.
The solid-state β→β+α transformation in titanium alloys can take place either diffusionless, i.e. in a martensitic process, or by a diffusion-controlled nucleation and growth process depending on cooling rate and alloy composition. This transition in transformation kinetics and kinematics enables the design of a huge range of very complex microstructures with features spanning across a broad range of length scales.
Consequently, thermo-mechanical processing is a useful method to tailor such microstructures and to control the size and morphology of the different phases in Ti alloys. Particularly, the control of the morphology of the α phase is one of the most important keys to improve the properties of α + β Ti alloys. The mechanical properties of two-phase (α + β) titanium alloys are very sensitive to the morphology and geometrical arrangement of the two phases, similar as in dual phase steels. A fully lamellar structure is characterized by high fatigue crack propagation resistance and high fracture toughness. Important parameters for a lamellar microstructure with respect to the mechanical properties are the β-grain size, the size of the colonies of α-phase lamellae, thickness of the α-lamellae and the nature of the inter-lamellar interfaces (β-phase). One of the most influential microstructural parameters is the α colony size, since the α colony size determines the effective slip length in lamellar microstructures. The cooling rate from the homogenization temperature determines the α colony size and width of the α-lamellae within the β grains and the extent of the continuous α-layer at β grain boundaries.
The unalloyed (pure) titanium crystalline structure undergoes an allotropic phase transformation from hcp (α – at lower temperature) to bcc (β – at higher temperature) by increasing the temperature up to 882°C. Consequently, alloying elements added to titanium are classified in two groups of α and β stabilizing solute alloy additions depending on whether they increase or reduce the α/β transformation temperature (882°C when unalloyed).
Alloying elements of titanium are typically grouped regarding their effect on the beta-transus temperature, hence, they are often termed as neutral, alpha-stabilizers, or beta-stabilizers. According to this classification scheme the alpha-stabilizing alloying elements extend the hexagonal alpha phase field to elevated temperatures, while beta-stabilizing elements shift the beta phase field to lower temperatures. Neutral elements have only minor influence on the beta-transus temperature. Apart from these targeted solute alloying elements that shift the equilibrium phase transition temperatures, there are also some nonmetallic elements on the order of few 100 ppm present in the form impurities, often from preceding synthesis or scarp usage when re-melting. Among the alpha-stabilizing elements aluminum is by far the most important alloying element of titanium. The interstitial elements oxygen, nitrogen, and carbon also belong to this category.
The role of elemental partitioning between β and ω phase in embrittling an originally ductile ω-containing Ti–12Mo (wt.%) model alloy was studied using transmission electron microscopy and atom probe tomography. It is revealed that the embrittlement of this alloy already occurs after aging at 400°C for as short as 10 min, when the size, inter-particle spacing and volume fraction of the ω particles remain almost unchanged. The origin of the aging-induced embrittlement is attributed to the significant rejection of Mo ( > 5 at.%) from the ω particles during aging, which leads to remarkable increase in the shear modulus ( > 30 GPa) of the ω particles, promoting intense plastic flow localization and facilitating crack nucleation prior to macroscopic yielding.
Neuer Datei-Download
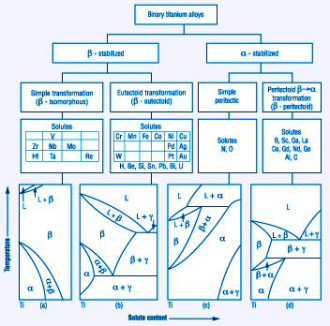
Beta Titanium: Solute Alloying Effects
β stabilizing elements: these are categorized into two groups of
(i) β isomorphous alloying elements (which are also referred to as fully β phase stabilizing elements) and
(ii) β eutectoid forming elements (which lead to a partially stabilized β phase) .
(i) β isomorphous elements are heavy refractory BCC elements such as Mo; V; W; Nb and Ta.
(ii) β eutectoid stabilizers are elements such as Fe; Cr; Mn; Co; Cu; Si and H.
There are some elements such as Zr, Hf and Sn which are neutral. They lower the α/β transformation temperature slightly and then increase it again at higher concentration.
This kind of titanium alloy is heat treatable and All β alloys contain small amount of aluminum which is an alpha stabilizer.
The most highly β stabilized alloys are alloys such as Ti-3Al-8V-6Cr-4Mo-4Zr and Ti-15V-3Cr-3Al-3Sn.
β alloys are exceedingly formable and they are not suitable for low temperature applications (unlike α-alloys which are suitable for cryogenic applications.)
Alpha-Beta Titanium Alloys: Solute Alloying Effects
α+β alloys: α+β alloys support a mixture of α and β at room temperature
They may contain (10-50)% β stabilizers at room temperature. If they contain more than 20% β stabilizers, the weld ability decreases. Because : On quenching – b decomposes to hcp martensite
Aluminum (Al) is added to the alloy as α-phase stabilizer and hardener due its solid solution strengthening effect. Vanadium (V) stabilizes ductile β-phase, providing hot workability of the
alloy.
The most important alpha-beta alloy is Ti-6Al-4V. High strength alpha-beta alloys include Ti-6Al-6V-2Sn and Ti-6Al-2Sn-4Zr-6Mo. They are stronger and more readily heat treated than Ti-6Al-4V.
Titanium α-β Alloys have high tensile strength and fatigue strength, good hot formability and creep resistance up to 425 ° C.
Aplications of Titanium Alloys
Aerospace applications
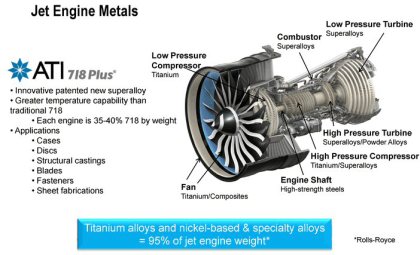
By combining light weight with high strength, titanium helps to reinforce airframes and enable higher performance in jet engines. In the case of the space shuttle, titanium is used for many critical parts, including the exterior paneling of the fuel tank and wing parts.
Aircraft and Jet Engines
Aircraft use a large amount of titanium alloy because it is light and extremely strong at high temperatures. Titanium is used to strengthen the frame structure and contributes towards the technical
advancement of jet engines.
Spacecraft
Titanium alloy, which has high corrosion resistance, high specific strength, and good heat resistance, is used for different spacecraft parts including outer fuel tank sheathing and wings.
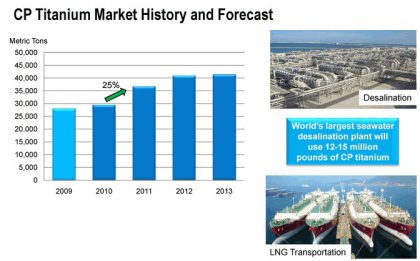
Chemical Industrial Production Plants
• LNG plants
• Seawater desalination plants
• Petroleum refineries
• Nuclear power plants
Recognized for total cost merits provided by its durability over an extended period, the adoption of titanium for plant structural and equipment materials is on the rise.
Tanker Trucks
Tanker trucks that carry sodium hypochlorite and sodium chromate use titanium because it is light, resistant to corrosion, and extremely strong.
Heat Exchangers
Titanium is a safe and economical material that is perfect for heat exchangers, which are used in extreme high-temperature and high-pressure conditions.
Biomedical applications
The main benefits of Titanium, enabling it as a primary implant material are:
+ Strength
+ Lightweight
+ Corrosion Resistant
+ Cost-efficient
+ Non-toxic
+ Biocompatible (non-toxic AND not rejected by the body)
+ Long-lasting
+ Non-ferromagnetic
+ Osseointegrated (the joining of bone with artificial implant)
+ Long range availability
+ Flexibility and elasticity rivals that of human bone
Two of the greatest benefits of titanium are its high strength-to-weight ratio and its corrosion resistance. Coupled with its non-toxic state and its ability to effectively withstand corrosion from bodi fluids titanium has become the main implant metal in the field of medicine.
Titanium is also very durable and long-lasting. When titanium cages, rods, plates and pins are inserted into the body, they can last for upwards of 20 years. And dental titanium, such as titanium posts and implants, can last even longer.
Another benefit to titanium for use in medicine is its non-ferromagnetic property, which allows patients with titanium implants to be safely examined with MRIs and NMRIs.
Osseointegration is a unique phenomenon where your body’s natural bone and tissue actually bond to the artificial implant. This firmly anchors the titanium dental or medical implant into place. Titanium is one of the only metals that allows for this integration.
With its excellent biocompatibility characteristics and low-occurrence of metal-related allergic reactions, titanium is widely used as a material for implants (artificial dental implants and artificial bone). Development of applications in other medical fields is expected.
Titanium Dental Tools
In the field of dentistry, titanium alloy, which is lighter than conventional steel, is now commonly used for tools. These titanium tools have higher corrosion resistance and strength than stainless
steel tools.
Artificial Dental Implants
Titanium has high biocompatibility and does not harm the human body. Therefore, it is ideal for use in dental implants.
Typical Examples of Commercial Titanium Alloys
Commonly used titanium alloys are:
Unalloyed Grades
ASTM Grade
ASTM Grade 2
ASTM Grade 3
ASTM Grade 4
ASTM Grade 7
Alpha and Near-Alpha Alloys
Ti-5Al-2.5Sn
Ti-8Al-1Mo-1V
Ti-6Al-2Sn-4Zr-2Mo
Ti-5Al-3Sn-3Zr-1Nb
Ti-5Al-4Sn-4Zr-1Nb
Ti-6Al-3Sn-4Zr-0.5Si
Alpha-Beta Alloys
Ti-6Al-4V
Ti-6Al-4V ELI
Ti-6Al-6V-2Sn
Ti-6Al-2Sn-4Zr-6Mo
Beta and Near-Beta Alloys
Ti-10V2Fe3Al
Ti-5Al-2Sn-2Zr-4Mo-4Cr
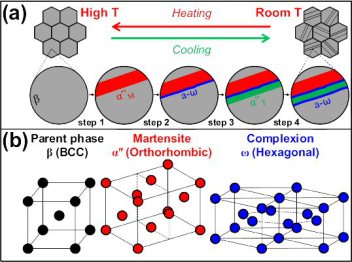
The most efficient way to tune microstructures and mechanical properties of metallic alloys lies in designing and using athermal phase transformations. Examples are shape memory alloys and high strength steels, which together stand for 1,500 million tons annual production. In these materials, martensite formation and mechanical twinning are tuned via composition adjustment for realizing complex microstructures and beneficial mechanical properties. Here we report a new phase transformation that has the potential to widen the application window of Ti alloys, the most important structural material in aerospace design, by nanostructuring them via complexion-mediated transformation.
Zhang 2017 Nature Communications Complex[...]
PDF-Dokument [6.7 MB]
Here we present a microstructure design approach which leads to partial recrystallization and nanoprecipitation within the same single-step heat treatment. This produces a dual-constituent microstructure.
Acta Materialia 135 (2017) 400 Partial [...]
PDF-Dokument [5.7 MB]
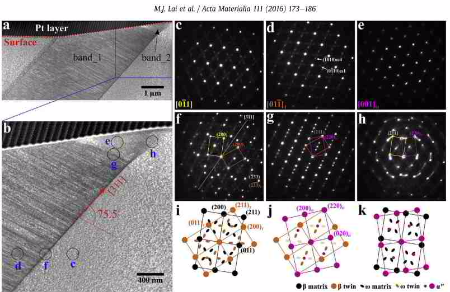
{332} twinning, an unusual twinning mode in other body-centered cubic (bcc) metals and alloys, has been demonstrated to be a fundamental deformation mode in bcc metastable beta titanium alloys:
On the mechanism of {332} twinning in metastable beta titanium alloys
Acta Materialia volume 111 (2016) pages 173 - 186
Twinning in metastable Titanium alloys A[...]
PDF-Dokument [2.4 MB]
{332} twinning, an unusual twinning mode in other body-centered cubic (bcc) metals and alloys, has been demonstrated to be a fundamental deformation mode in bcc metastable beta titanium
alloys. Recent studies suggest that this twinning mode plays an important role in enhancing the work hardening and
thus improving the mechanical properties. Here, we studied the mechanism of this twinning mode in a metastable beta Ti - 36Nb - 2Ta - 3Zr (wt.%) alloy. Tensile tests were performed to induce the
formation of {332} twins. By using electron backscatter diffraction, transmission electron microscopy and in situ
scanning electron microscopy, the surface-to-bulk microstructures and the initiation and propagation of {332} twins were investigated. In addition to the previously reported high densities of
straight dislocations within the twin, we have observed that an a'' martensite band is present near the surface
adjacent to the twin. During annealing at 900°C, the a'' martensite band transforms into the adjacent twin rather than into the matrix, indicating that {332} twin nucleates within a''
martensite. Further evidence for this is the constitution of the twin in the initial stage of its formation, where the first portion formed consists of a'' martensite. During propagation,
the twins propagating to the opposite directions can merge together when their lateral boundaries impinge on each other. Based on the experimental observations, an a''-assisted twinning
mechanism is proposed and the origin of the dislocations within {332} twin is discussed accordingly.
Acta Materialia vol 113 (2016) page 311-319
Acta Materialia vol 113 (2016) 311 Titan[...]
PDF-Dokument [2.2 MB]
In this project we have computed formation energies for all technologically relevant transition metal solutes in the alpha, beta, and omega phases of Ti, employing ab initio simulations. We
analyze and explain their periodic-table trends, and from their differences we derive stabilization energies which provide direct insight into phase stabilization effects of the various
solutes with respect to alpha, beta, and omega. This allows us to identify strong beta stabilizers in the middle of each electronic d shell in consistency with experimental knowledge. Based
on an extension of the stabilization energies to free energies we derive a wide range of Ti-transition metal phase diagrams. A detailed comparison to available experimental martensitic
transformation temperatures and to measurements performed in this study shows that, despite some quantitative discrepancies, the qualitative trends can be expected to be correct. An important
feature that is displayed by a limited range of the computed phase diagrams is a triple point at which the three phases, alpha, beta, and omega, meet. This
insight provides a plausible explanation for the complexity observed in gum metals, a class of Ti alloys with very special materials properties.
Acta Materialia 97 (2015) 291-304
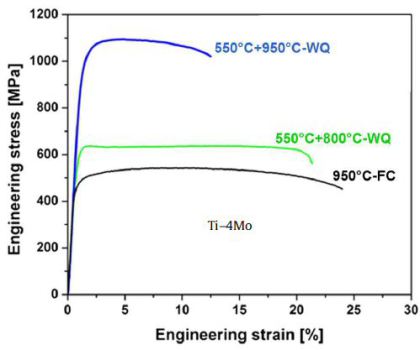
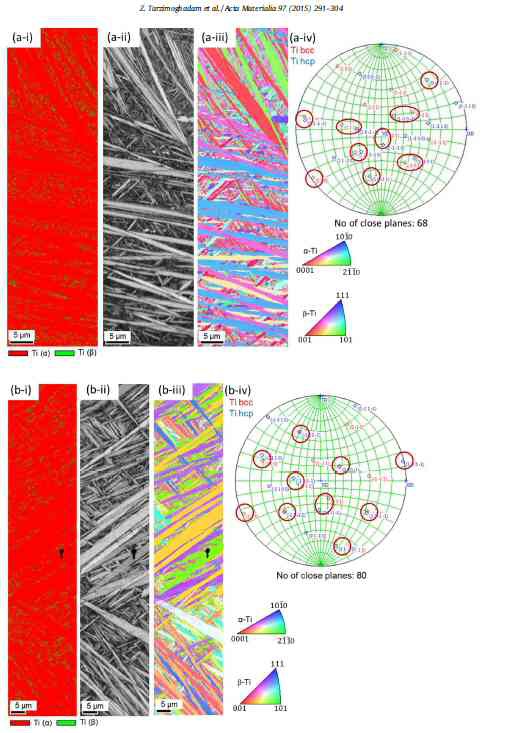
Microstructure design and mechanical properties in a near-alpha Ti–4Mo alloy
Z. Tarzimoghadam, S. Sandlöbes, K.G. Pradeep, D. Raabe
Acta Materialia 97 (2015) 291 Ti Mo Micr[...]
PDF-Dokument [3.8 MB]
We study the effects of different heat treatment routes on microstructure engineering and the resulting mechanical response in a plain binary Ti–4Mo (wt%) model alloy. We observe a broad
variety of microstructure formation mechanisms including diffusion driven allotropic phase transformations as
well as shear and/or diffusion dominated modes of martensitic transformations, enabling a wealth of effective microstructure design options even in such a simple binary Ti alloy. This wide
variety of microstructures allows tailoring the mechanical properties ranging from low yield strength (350 MPa) and high ductility (30–35% tensile elongation) to very high yield strength
(1100 MPa) and medium ductility (10–15% tensile elongation) as well as a variety of intermediate states.
Mechanical testing and microstructure characterization using optical microscopy, scanning electron microscopy based techniques, transmission electron microscopy and atom probe tomography were
performed revealing that minor variations in the heat treatment cause significant changes in the resulting microstructures (e.g. structural refinement, transition between diffusive and
martensitic transformations). The experimental results on microstructure evolution during the applied different heat treatment routes are discussed with respect to the mechanical properties.
Acta Materialia 55 (2007) 4475-4487
Theory-guided bottom-up design of b-titanium alloys as biomaterials based on first principles calculations: Theory and experiments
D. Raabe, B. Sander, M. Friak, D. Ma, J. Neugebauer
Acta Materialia 55 (2007) 4475–4487 ab i[...]
PDF-Dokument [1.9 MB]
Here we present a new strategy for the theory-guided bottom up design of beta-Ti alloys for biomedical applications using a quantum mechanical approach in conjunction with experiments.
Parameter-free density functional theory calculations are used to provide theoretical guidance in selecting and optimizing Ti-based alloys with respect to three constraints: (i) the use of
non-toxic alloy elements; (ii) the stabilization of the body centered cubic b-phase at room temperature; (iii) the reduction of the elastic stiffness compared to
existing Ti-based alloys. Following the theoretical predictions, the alloys of interest are cast and characterized with respect to their crystallographic
structure, microstructure, texture, and elastic stiffness. Due to the complexity of the ab initio calculations, the simulations have been focused on a set of binary systems of Ti with two
different high melting body-centered cubic metals, namely, Nb and Mo. Various levels of model approximations to describe mechanical and thermodynamic properties are tested and critically
evaluated.
The experiments are conducted both, on some of the binary alloys and on two more complex engineering alloy variants, namely, Ti–35 wt.% Nb–7 wt.% Zr–5 wt.% Ta and Ti–20 wt.% Mo–7 wt.% Zr–5 wt.%
Ta.
What is the role of the omega phase in Titanium alloys ?
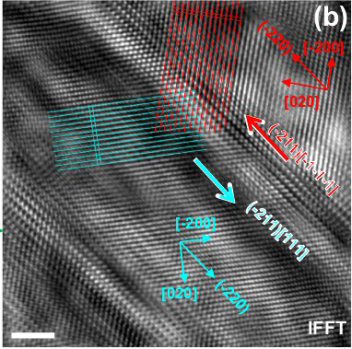
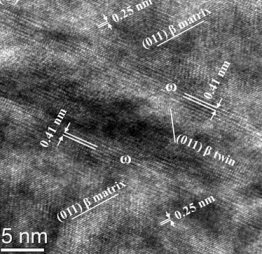
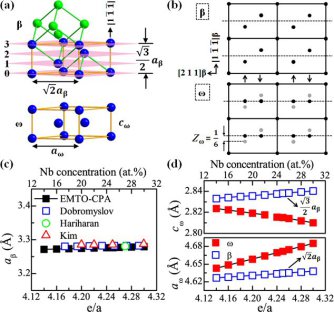
Ti-Nb-based alloys are essential materials for biomedical implant and aerospace applications. They reveal complex phase transformation behavior. In our studies, we investigated the {211}β<111>β twinning induced β (body-centered cubic phase) to ω (hexagonal phase) transition in Ti-Nb-based alloys by transmission electron microscopy and ab initio calculations in conjunction with the linear elastic inclusion theory. Our theoretical results reveal a distinct energy barrier for the β to ω transition, where the contribution from lattice rearrangement, rather than the elastic contribution associated with lattice mismatch, plays the major role. It is shown that this energy barrier can be overcome by {211}β<111>β shear, explaining why {211}β<111>β twinning or, alternatively, the β to α’’ transition promotes local formation of the ω phase.
Why are metastable phases in Titanium alloys important ?
Improved understanding of the metastable ω phase in β-type Ti alloys and Zr alloys is important as it affects the mechanical and physical properties of the material. Several works documented the various ω phase morphologies and underlying mechanisms.
Why phase transformation pathways can occur between beta and omega in Titanium alloys ?
There are various types of ω phase morphologies and associated transformation mechanisms: the β to ω transformation can occur
(i) thermally, i.e. via rapid cooling from the single body-centered cubic (bcc) β phase field or by subsequent isothermal aging, producing ellipsoidal or cuboidal ω particles homogeneously distributed throughout the β matrix; or
(ii) mechanically, i.e. via extremely high strain-rate compressive loading, producing non-uniformly distributed ω plates (hereafter referred to as plate-like ω).
Interestingly, recent studies on Ti-Nb-based alloys4-6 report similar plate-like ω phase during quasi-static compression (in the vicinity of the {211}β<111>β twin boundary) even during quenching (at the α’’/β interface).
Considering that both twinning and α’’ transformation involve a shear process, it can be assumed that the formation of the plate-like ω phase is due to the atomic shear rather than compressive stress state. This assumption, however, is so far not fully confirmed in the literature. Moreover, for the compositions listed in Table I (valence electron number e/a = 4.22 ~ 4.24), previous ab initio investigations8 show that the ω phase is more stable compared to the β phase, anticipating a spontaneous transition from β to ω, in contrast to the experimentally observed need for shear-assistance. Taking into account that the formation of an ω inclusion is concerned with lattice mismatch and the phase transformation is a kinetic process, it can be hypothesized that the atomic shear may have either one of two roles: decreasing the elastic strain energy associated with lattice mismatch between β and ω, or overcoming a kinetic barrier existing on the energetic pathway. This is yet another aspect that is not fully clarified.
Twinning in metastable Titanium alloys A[...]
PDF-Dokument [2.4 MB]
Acta Materialia 2018 dislocation channel[...]
PDF-Dokument [3.5 MB]
PDF-Dokument [6.0 MB]