Quench and partitioning (Q&P) steels
Basic research on advanced high strength steels is challenged by an increasing and urgent demand for designing and synthesizing high-strength alloys that enable the manufacturing of highly fuel-efficient vehicles with maximum passive passenger safety.
One essential type of high-strength steel for such applications in car bodies are the transformation-induced plasticity (TRIP) type grades. These steels contain retained, patrially stable austenite and hence show excellent ductility. However, the tensile strengths of conventional low-carbon TRIP steels with microstructures consisting of ferrite, carbide-free bainite, retained austenite and small amounts of martensite generally do not exceed 1100 MPa. This limitation can be attributed to the fact that their ferrite volume fraction is normally too high so as to accumulate a sufficient amount of carbon into austenite during intercritical annealing and following austempering to obtain stable retained austenite. In order to achieve a higher strength level above 1100 MPa while maintaining high ductility, several novel steels that utilize retained austenite have been developed in the last decade, such as nanocrystalline bainitic steel (or super bainite), maraging-TRIP steel and quenching and partitioning (Q&P) steel.
Q&P steels yield an excellent balance of high tensile strength and good elongation with similar chemical compositions as conventional TRIP steels. They are produced via the
Q&P process which consists of a quenching
and a partitioning step. In the quenching step, fully austenitized or intercritically annealed steels are quenched to temperatures (hereafter referred to as the “quench temperature”)
below the martensite start (Ms) temperature but above the martensite finish (Mf) temperature in order to form a controlled volume fraction of martensite. The quenched steels are then
held at the same or higher temperatures than the quench temperature during the subsequent partitioning step. Austenite that prevails after quenching is considered to be stabilized
through carbon partitioning from martensite into the austenite during the partitioning treatment. The resultant microstructures of the steels mainly consist of tempered martensite and
retained austenite so that a higher strength can be achieved as compared to conventional TRIP steels.
Acta Materialia 65 (2014) 215-228
Atomic-scale analysis of carbon partitioning between martensite and austenite by atom probe tomography and correlative transmission electron microscopy
Acta Materialia 65 (2014) 215 carbon par[...]
PDF-Dokument [1.7 MB]
Carbon partitioning between ferritic and austenitic phases is essential for austenite stabilization in the most advanced steels such as those produced by the quenching and partitioning
(Q&P) process. The atomistic analysis of the carbon partitioning in Q&P alloys is, however, difficult owing to the simultaneous occurrence of bainite transformation, which can also
contribute to carbon enrichment into remaining austenite and hence overlap with the carbon partitioning from martensite into austenite. Therefore, we provide here a direct
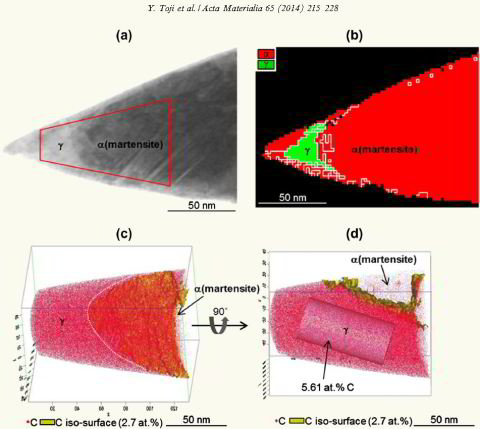
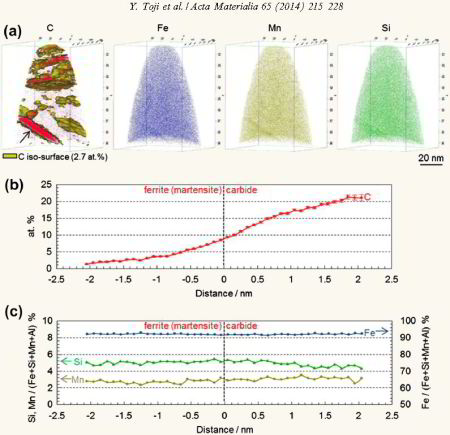
atomic-scale evidence of carbon partitioning from martensite into austenite without the presence of bainite transformation. Carbon partitioning is investigated by means of atom probe tomography and
correlative transmission electron microscopy. A model steel (Fe–0.59 wt.% C (2.7 at.% C)–2.0 wt.% Si–2.9 wt.% Mn) with martensite finish temperature below room temperature was designed and
used in order to clearly separate the carbon partitioning between martensite and austenite from the bainite transformation. The steel was austenitized at 900 °C, then water-quenched and tempered
at 400 °C. Approximately 8 vol.% retained austenite existed in the as-quenched state. We confirmed by X-ray diffraction and dilatometry that austenite decomposition via bainite transformation did
not occur during tempering. No carbon enrichment in austenite was observed in the as-quenched specimen. On the other hand, clear carbon enrichment in austenite was observed in the 400 °C
tempered specimens with a carbon concentration inside the austenite of 5–8 at.%. The results hence quantitatively revealed carbon partitioning from martensite to austenite, excluding bainite
transformation during the Q&P heat treatment.
Acta Materialia 86 (2015) 137-147
Carbon partitioning during quenching and partitioning heat treatment accompanied by carbide precipitation,
Acta Materialia 86 (2015) 137 Toji carbo[...]
PDF-Dokument [1.8 MB]
Quenching and partitioning (Q&P) steels yield an excellent balance of high tensile strength and good elongation, with chemical compositions similar to conventional TRIP steels.
They are produced via the Q&P process, which
consists of a quenching and a following partitioning step. During the quenching step, fully austenitized or intercritically annealed steels are quenched to temperatures (hereafter
referred to as “quench temperature”) below the
martensite start (Ms) temperature, but above the martensite finish (Mf) temperature in order to form a controlled volume fraction of martensite. The quenched steels are then held at
temperatures the same as or higher than the quench temperature during the subsequent partitioning step. Austenite that prevails after quenching is considered to be stabilized through
carbon partitioning from martensite into austenite during the partitioning treatment.
It has been suggested that the carbon partitioning from martensite into austenite is controlled by the constrained carbon equilibrium (CCE) criterion. This criterion aims to predict
the carbon concentration in austenite under the condition where: (i) competing reactions, such as cementite or transition carbide formation or bainite transformation, are suppressed;
(ii) an identical carbon chemical potential
exists in both ferrite (or martensite) and austenite; and (iii) the carbon partitioning proceeds under the assumption that the interface between ferrite and austenite does not migrate.
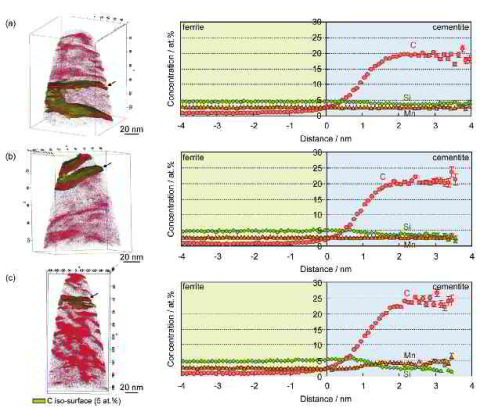
However, carbide precipitation in martensite is often observed during the partitioning step, even in low-carbon steels as well as in high-carbon steels, even if they contain a high
amount of Si. If carbide precipitates, some of the carbon is consumed to form the carbide, reducing the remaining amount of carbon in martensite that can be enriched in austenite
during partitioning.
Hence, the austenite carbon concentration after the partitioning step in this case is presumed to be lower than that predicted under the CCE conditions excluding carbide
precipitation. As Speer pointed out, it is important to choose appropriate chemical compositions in order to avoid carbide precipitation in realizing an ideal Q&P
condition. However, as some carbide formation may always occur, adequate models are required that describe such a case, thus providing a more precise estimate of the
carbon concentration in austenite after the Q&P heat treatment. There is, however, currently no model dealing with the carbon partitioning behavior from martensite into
austenite under conditions in which carbide precipitation occurs in martensite during the partitioning step. Therefore, this study conducts an experimental analysis of the carbon
partitioning behavior from martensite into austenite accompanied by carbide precipitation inside the
martensite during a partitioning step. A modified CCE model is introduced to explain the experimental results.
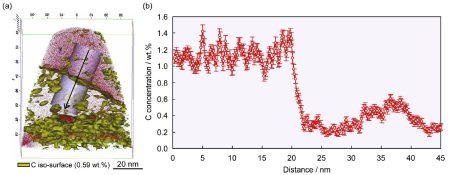
More specific, since the carbon partitioning from martensite into austenite in the quenching and partitioning (Q&P) process has been suggested to be controlled by the constrained carbon equilibrium (CCE) criterion we study here the details of this mechanism. The CCE model defines an approach for predicting the carbon concentration in austenite under the condition that competing reactions such as carbide formation and bainite transformation are suppressed. Carbide precipitation in martensite is, however, often observed during the partitioning step, even in low-carbon steels as well as in high-carbon steels, even when containing a high amount of Si. Therefore, carbon partitioning from martensite into austenite is studied here, considering carbide precipitation in martensite. Carbon partitioning was investigated by means of a field-emission electron probe micro analysis (FE-EPMA) and atom probe tomography (APT), using 1.07 wt.% and 0.59 wt.% carbon steels with various martensite volume fractions. Carbon partitioning from martensite to austenite was clearly observed in all specimens, even though a considerable amount of carbide precipitated inside the martensite. The austenite carbon concentration after the partitioning
step was not influenced by either the martensite volume fraction or the bulk carbon content. A modified model for predicting the austenite carbon concentration after the partitioning step was
proposed to explain the experimental results by assuming carbon equilibria between austenite,
ferrite and cementite under a constrained condition.